By Rene Russo, CEO of Xilio Therapeutics, as part of the From The Trenches features of LifeSciVC
When your team’s priority is to execute a well-formed plan, then a “heads down” focus is critical. We all recognize this situation, using it to drive project management, accountability, and collaboration.
The opposite priority, the “heads up” focus, encourages your team to observe the levels of employee engagement across a company’s changing “external” landscape. This approach lets you see the white space and determine how the company and employees mesh together.
What about the situation when the plan isn’t yet fully formed? The external environment is changing rapidly. Our industry (and our world) still faces substantial disruption from a global pandemic. Even so, the team still needs to deliver on a big vision for the patients, investors, partners, employees, communities we serve. Many of these concepts were covered in the still very relevant “State of the Industry – 2021 Year in Review”.
In our situation at Xilio, the “heads up and eyes wide open” approach is essential to know how to anticipate, plan, get creative, manage risks, and pivot when needed.
I have written often about our long-range planning process at Xilio both in terms of engaging the entire company, and ensuring that we take the time to align on priorities. By having our company’s full engagement in defining our future, I have been able to collaborate with many talented colleagues across multiple disciplines, deciding together how to deliver on our vision. As one of our employees shared about this full company experience “We are agile, practical, and embrace change positively, avoiding unnecessary bureaucracy. We tackle the right priorities in an organized, but always nimble and empowered manner.”
Our most recent adaptation of this planning process is now called XSTAR, or Xilio Strategic Thinking and Roadmap. We have elevated the approach beyond the financial components of Long-Range Planning (LRP) to include Functional Planning with each team owning their own capability build and Portfolio Planning and Prioritization to ensure we understand the diseases we hope to impact with our novel science.
This past year, we evolved into a clinical-stage company, advancing two tumor-selective immunotherapy programs into the clinic in parallel, all while continuing to accelerate our discovery pipeline and weathering a global pandemic that disrupted all parts of the drug development continuum. We focused on a “heads up and eyes wide open” approach to understanding the external trends that will impact our ability to deliver on our programs. It is my strong belief that we need to build a company that will thrive in an uncertain future – so knowing how the world is changing is as impactful as listing benchmarks that illustrate where we have been.
This From the Trenches blog’s focus is to come out of the trenches and look up and at the trends affecting us all. My hope is we can collectively engage in ways to optimize our efforts to deliver our life-saving medicines to patients in the changing external environment.
Some trends are incredibly progressive: faster decision making, decentralized reporting, and increasing focus on inclusion.
Other trends are very challenging: the war for talent, global supply chain disruptions, and the external partners’ limits.
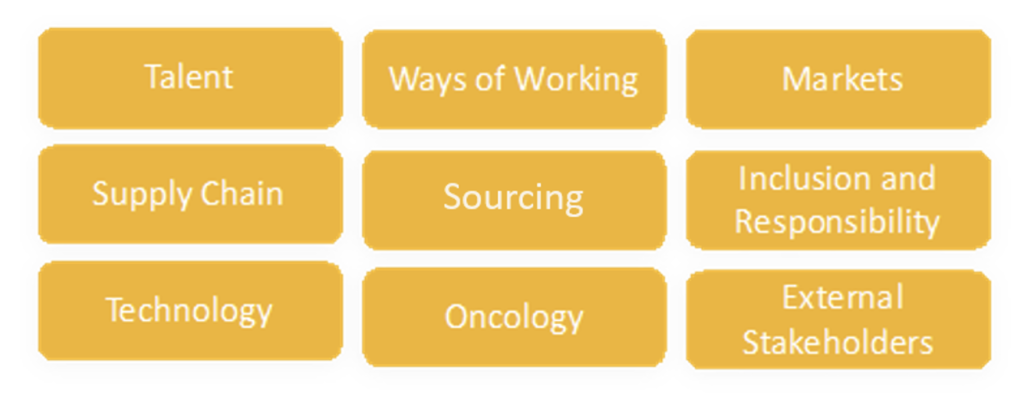
After we heard from all of Xilio’s teams about their discipline’s external trends, we synthesized those insights into the following categories:
Streamlining the details to capture the essence, the following synthesis represents the trends with the highest impact on our collective work. These results also warrant additional discussion at both the industry and company levels:
External Trends Early 2022 Summary:
- Talent
- Employees caught within the biotech industry’s talent war hold new expectations of companies around growth/development, compensation, corporate social responsibility, and exposure as well as the required qualifications/experience for roles.
- States will compete to attract individual talent rather than trying to get companies to relocate. Both points were noted in recent media reports about the shortage of workers in our booming biotech industry. Our team’s focus on capability-building was prescient last year but is now critical to ensure we can deliver on our goals.
- Supply Chain
- COVID-19 continues to impact supply chains across the biotechnology and drug development spectrum, highlighting the need for alternative sourcing options to ensure business continuity.
- Increased transparency and visibility across the digitized supply chain and personalized treatments means that inventory management will become more tightly focused.
- Technology
- Technology’s ability to link machine learning that detects patterns in complex data with modeling can provide insights into what the patterns mean.
- This creates the capability to make predictions for subgroups or even individual patients based on their specific patterns.
- High throughput systems and processes allow teams to make decisions faster and increase pressure in a competitive environment which can impact expectations regarding timelines
- “Almost all aspects of innovation in the areas of technology are increasing the speed of decision-making which requires our teams to adapt as a routine way of operating”
- Ways of Working
- Tools and technology improvements continue to evolve, requiring new ways of working. We discussed this in our Aug 2020 article on Zooming in on What Matters. As an industry, we have only become more reliant on technology and tools to enhance our collaboration.
- COVID-19 has accelerated the focus on technology for centralized and indirect (remote) clinical trial monitoring due to limited site staff’s availability and limited access to trial sites.
- Systems’ integration improves efficiency, completeness, and accuracy of information.
- This integration plus improved knowledge and document sharing are essential for optimal productivity and functional performance, including more flexible and agile solutions.
- “We see a blurring of internal functions as data become more accessible through dashboards and systems integration; HR and legal, IT and finance, digital communications to accelerate trial recruitment”.
- Sourcing
- For some years, we’ve seen substantial growth of the market for clinical research organizations (CRO) and contract manufacturing organizations (CMO) market. They have been constrained and consolidating, resulting in a few larger organizations playing a significant role and shaping the industry. Capacity was constrained, and as with all major stakeholders, relationships mattered.
- COVID and more recent geo-political risks have forced a reassessment of these models, with an enhanced focus on business continuity. For some activities, such as protein sciences and process development, biotechnology companies are looking at bringing them back inside where possible. For other activities, many companies are focusing on potentially partnering with smaller CROs.
- Oncology
- For oncology, COVID and labor shortages are having a continued impact on most industries, and life sciences is no exception. This particularly impacts patients, as many are unable to have procedures that are not a priority.
- Actual clinical care is the priority for patients, so labor shortages are most acutely felt in the research setting. Across the oncology spectrum, we are seeing clinical trial sites decline to open due to a lack of personnel in roles such as pharmacy and nursing.
- There is also a growing concern in the regulatory environment of the number of accelerated approvals in recent years. The failure of many confirmatory trials has led the FDA to reconsider the threshold for accelerated approval and require randomized trials comparing new therapies to a relevant standard of care for approval.
- Markets
- Companies are going public earlier, resulting in a more competitive financing market and a different approach to building finance teams.
- Alternative financing and transacting structures continue to evolve, leading to new options (e.g., co-promotion, hybrid splits, and equity licensing constructs) for strategic consideration.
- This is all taking place against a backdrop of challenging biotech capital markets conditions with the XBI down nearly 50% from its early 2021 highs and a majority of recent IPO’s trading below their IPO price as markets digest significant infusions of capital and a significant increase in public listings.
- Inclusion and Responsibility
- Companies and executive leadership are increasingly being held accountable for corporate practices that promote good stewardship as well as increasing the focus on environmental, social, and corporate governance (ESG).
- There is a clear focus on inclusivity and diversity in protocols to ensure that trials are designed to fulfill market/regulatory requirements worldwide while also serving diverse patient populations.
- External Stakeholders
- An increasing number of companies with multiple assets are putting strain on external stakeholders, including regulatory authorities, which risks decreased interactions or engagement over the course of drug development.
- The bar for value among payers has changed, increasing the burden of proof around substantial differences in outcomes and adoption on guidelines.
In our long-range planning process, we are building our capabilities, defining our processes, and prioritizing our resources with these trends in mind. Most importantly, we are engaging the brilliant minds in our company to see the future and ensure we are serving our patients in it.
As an industry, we need to find forums to address how these trends are both accelerating and impeding our ability to deliver on our promise for patients. Let me ask you this:
- What ways do you think we can manage some of the challenges together that are defined by these trends?
- What forum should we use to identify those challenges to develop a plan that allows all of us to deliver on our commitment to patients?
My hope for our cross-industry collaboration is that we can operate in the way that one of our employees recently described;
“We are all problem-solvers coming together like swarms of bees to get things done. The spirit of collaboration and cooperation is uplifting – even during the Covid-19 era”.
I’d love to hear from you, so please, reply here with any of your ideas