This blog was written by Mike Gilman, CEO of Obsidian Therapeutics and Atlas Advisor, as part of the From The Trenches feature of LifeSciVC.
Consider the leech – the first living medicine. In its heyday in the 19th century – France imported forty million leeches a year for medical use in the 1830s– bloodletting by leeches was the go-to treatment for whatever ailed you. It may not have helped much, but it probably didn’t do much harm either.
 Now consider the CAR-T cell. As leeches packaged up a bunch of complex biology that early physicians put to questionable use for their patients, CAR-T cells bottle all of the powerful biology of a living T cell and unleash it on a patient’s tumor. Unlike leeches, though, which are basically gross and useless, CAR-T cells are elegant and breathtakingly powerful. But they exist on a therapeutic knife edge, teetering between profound efficacy and brutal toxicity. We can surely see all the good they can do, the lives they will save, but they need to be tamed. And if we can do that – if we can turn CAR-T cells into an everyday medicine that can be managed precisely and dynamically like any other drug – then perhaps these therapies can become available to any patient with cancer. Imagine the lives we could save.
Now consider the CAR-T cell. As leeches packaged up a bunch of complex biology that early physicians put to questionable use for their patients, CAR-T cells bottle all of the powerful biology of a living T cell and unleash it on a patient’s tumor. Unlike leeches, though, which are basically gross and useless, CAR-T cells are elegant and breathtakingly powerful. But they exist on a therapeutic knife edge, teetering between profound efficacy and brutal toxicity. We can surely see all the good they can do, the lives they will save, but they need to be tamed. And if we can do that – if we can turn CAR-T cells into an everyday medicine that can be managed precisely and dynamically like any other drug – then perhaps these therapies can become available to any patient with cancer. Imagine the lives we could save.
Now, think about all of the potent biology wrapped up in other cells in our bodies. How can we capture and tame them as well, so that we can put the biology of living cells to therapeutic use even more broadly? That is the amazing new world of living medicines ahead of us, if we learn how to bring cells under control.
Today, I want to tell you how Obsidian Therapeutics plans to do precisely that by building pharmacologic operating systems for living medicines.
First, some ancient biotech history
My first industry job, starting in 1994, was at ARIAD Pharmaceuticals, then a two-year-old startup. My job was to set up and run a team to develop a new idea from Stuart Schreiber at Harvard and Jerry Crabtree at Stanford. Stuart and Jerry, along with the talented folks in their lab, showed that they could use bivalent derivatives of FKBP12 ligands, initially two molecules of FK506 joined by a linker (which they imaginatively named FK1012) to reversibly dimerize engineered fusion proteins via these FKBP12 domains and, by doing so, to bring various biological activities under small(ish)-molecule control.
Our job at ARIAD was to humanize and ruggedize the system for clinical use in gene and cell therapy. Over the course of several years, we built a transcriptional control system in which we used dimerizers (ultimately rapamycin and derivatives) to conjoin an engineered DNA-binding domain with a companion transcriptional activation domain, assembling an active transcription factor that acted at a synthetic promoter. That work culminated in the generation of monkeys that were injected with AAV vectors expressing the engineered transcription factors and a target gene encoding EPO. For years, those animals could be administered a dose of rapamycin and would respond with a blast of circulating EPO and a bump in hematocrit. We also built a suicide switch for use in T cells, in which a dimerizer molecule induced multimerization of a transmembrane protein containing the signaling domain of the Fas receptor, triggering apoptosis.
It all worked beautifully.
Except that we were solving a problem that no one thought they had. In those early days of gene therapy, it was a challenge getting any detectable transgene expression at all. So controlling that expression was not on anyone’s worry list. And cell therapy? Well, no one was taking that seriously at all.
And then, sadly, came the death of Jesse Gelsinger in a gene therapy trial, and the entire field went dark for a decade.
So the ARIAD program was eventually shuttered and the technology was licensed out to a reagent company. But it was also licensed to a new startup out of Baylor, which became Bellicum, now an important company in the cell therapy space.
Back in the early days of this program at ARIAD, a graduate student in the Schreiber lab and a postdoc in the Crabtree lab did much of the work on this system. The postdoc in the Crabtree lab was David Spencer, who founded Bellicum out of Baylor and is now Bellicum’s CSO. The student in the Schreiber lab was Tom Wandless, who went to Stanford and tinkered resolutely with the technology for twenty years.
About two years ago, Tom decided that a new twist on the technology was ready for prime time and wanted to figure out how to spin it out into a company. To figure out how to do it, he decided to try to track me down. Ever the resourceful guy, he sent an email to the “info” address on the website of my company at the time, Padlock Therapeutics. And lest you think those links are black holes, I actually got the message and got on the phone with him. He explained what he was doing. I thought it was cool and a significant leap over the dimerizer approach. And with gene and cell therapy roaring back, now was the time to put this technology to work. I introduced Tom to the folks at Atlas Venture. They got excited too and decided to found a company with Tom. Obsidian Therapeutics was born in late 2015 and became operational in early 2016.
That was the end of my involvement as I had my own company to run. But after the Padlock acquisition in April 2016, I agreed to join the Obsidian board. And after utterly failing at retirement, I joined as CEO in October of that year. I think the reader can perhaps appreciate why. Rare and precious is the opportunity to take a second crack at a problem you had to walk away from the first time around.
How to control almost anything in a cell
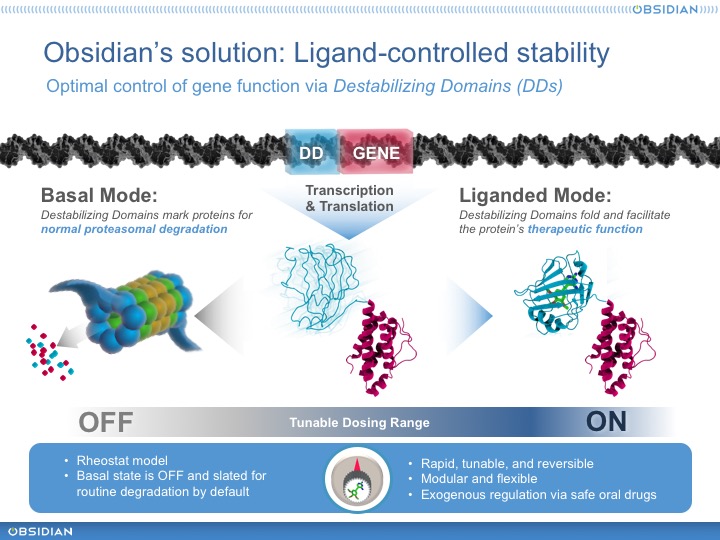
What did Wandless figure out, exactly? Well, as he continued to work with FKBP12, he discovered a particularly interesting class of mutants. These proteins were conditionally stable. That is, when initially produced they were unstable – rapidly ubiquitinated and degraded by the proteasome, presumably because they are partially unfolded. But when provided with a small molecule ligand, they would fold right up and become stable. Moreover, because the proteasome is processive, these destabilizing domains (or DDs, as we’ve come to call them) could confer conditional stability on a fused heterologous domain. In other words, it was possible to control the stability of virtually any payload protein, sensitively and precisely, with a small-molecule compound. He quickly exemplified one of the many killer apps for this technology, showing that he could precisely control secretion of a cytokine (IL2) in cells and in animals.
This is Obsidian’s founding technology and over the last couple of stealthy years, we have reduced it to practice in several important ways. First, we’ve discovered new human DDs whose stability we can control with safe, orally-available, and well-understood marketed drugs. No NCEs or potent rapamycin derivatives. Instead, off-the-shelf, mostly generic drugs that physicians can prescribe and dispense today. Moreover, we’ve built a powerful toolkit to rapidly and reproducibly build and optimize DD cassettes – synthetic biological units encoding DD-controlled proteins that can be installed in a vector and provide an “operating system” for the engineered cell. And, of course, we’ve built some pretty interesting product prototypes.
New CARs with new controls
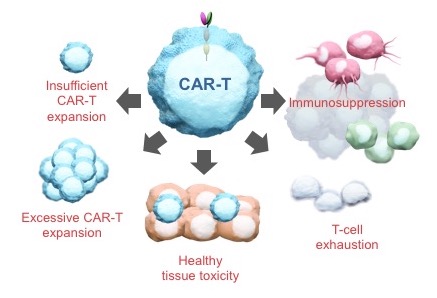 Let’s come back to CAR-T cells. We know what they can do today. Imagine, though, that you now have the ability to equip these cells with new functions that are under precise and dynamic pharmacologic control.
Let’s come back to CAR-T cells. We know what they can do today. Imagine, though, that you now have the ability to equip these cells with new functions that are under precise and dynamic pharmacologic control.
We have. And here are a few things we’re building.
Think about what happens when a cancer patient receives an infusion of CAR-T cells. First of all, these patients have been “preconditioned” – a euphemism for a nasty program of chemotherapy to burn out their existing hematopoietic system to “make room” for the incoming therapeutic cells. What does that mean? Mainly, it means the patients will have responded homeostatically to depletion by cranking up a bunch of proliferative cytokines like IL15, which is pretty much the first thing the infused cells see and to which they respond accordingly. Soon enough they encounter antigen on tumor cells and are further stimulated to proliferate through their CAR. In short order, the cells expand explosively and uncontrollably. Within days, most patients experience a cytokine storm that puts many of them in the ICU. Most make it through – and those who do can experience dramatic and previously undreamt-of anti-tumor responses – but, sadly, some don’t.
The problem here is that once the cells are administered to the patient, the physician surrenders all control. The cells are on their own. We can only respond to the havoc they create and hope for the best.
What if, instead, we could control the proliferation of cells directly? We could, for example, control expression of the CAR on the cell surface and thereby modulate the degree of antigen-stimulated proliferation. We could start the cells with receptor expression low, dose-escalate in an orderly fashion until we see a clinical signal, and then hold the dose there or dial it back for the duration of treatment. That is certainly not an unfamiliar scenario for cancer docs – it’s how many anti-cancer drugs are dosed. Moreover, like other cancer treatments, we could cycle therapy on and off, giving both the cells and the patients a periodic rest, which is likely to lead to more tolerable and durable therapy.
And what if we could equip CAR-T cells with a cell-autonomous antigen-independent proliferative engine that we could dial up and down at will? That would allow the physician to keep the therapeutic cells alive and active even after antigen-driven signaling has subsided. That’s important because persistence of the therapeutic cells is critical to long-lasting remissions or cures.
Maybe we could dispense with brutal preconditioning regimens entirely and drive incoming cells into the host with an internal IL15-like signal. If we can eliminate preconditioning as a prerequisite for adoptive immunotherapies, then these therapies can be made available to patients who are not sick enough – or too sick – to suffer through preconditioning.
What if we could give these cells superpowers to help them do their job more effectively. Consider, for example, IL12, a cytokine widely admired for its ability to stimulate anti-tumor immunity in solid tumors but just as widely feared for its lethal toxicity. Augmenting CAR-Ts with IL12 is pretty much a non-starter if it’s not under very tight control.
These are some of the actual products in development at Obsidian. Our whiteboards are filled with many more ideas – and that’s just for novel next-generation CAR-T products. But the list grows exponentially when you start thinking about harnessing the biology of other immune system cells – particularly cells with naturally short half-lives or those that are challenging to expand ex vivo, where a DD-regulated proliferation signal could enable them to persist long enough to complete their mission. And with the expanded use of cell and progenitor cells as substrates for gene modification, we think it will be possible to dynamically drive genetically modified progenitor cells down a lineage of interest by controlling the activity of an appropriate regulatory factor. With the ability to add new functionality to living cells and to control these functions precisely and dynamically with simple small-molecule drugs – to build operating systems into these cells – a new world of living medicines awaits.
We’re off
I think you can see why we’re excited by the opportunities in front of us at Obsidian. And investors are excited too. We’ve been in classic stealth mode for nearly two years, largely funded by Atlas Venture with help along the way from Alexandria Venture Investments. That’s allowed us to advance the technology platform, build some exciting prototypes, assemble an outstanding team, and move into some great space in Cambridge. And today we can tell you that some fantastic new investors have joined the party. We’ve just closed a $49.5M Series A round, led by the visionaries at GV (the entity formerly known as Google Ventures, the venture capital arm of Alphabet, Inc.), with participation from Takeda Ventures, Vertex Ventures, Amgen Ventures, and ShangPharma Innovation, along with our early believers, staunch supporters, and seed investors at Atlas and Alexandria.
We’ve also announced an important research collaboration with Crystal Mackall at Stanford. Dr. Mackall and her colleagues have already collaborated with Tom Wandless to show that DDs make it possible to modulate CAR expression in T cells and that doing so alleviates the functional exhaustion caused by chronic CAR signaling. Now she will install our fully-developable DDs into CAR vectors with the goal of creating novel CAR-T products that can be taken to patients.
Timing is everything in our business. We had fantastic technology back at ARIAD in the 1990s but no one needed it. Now, Obsidian has even better technology – simple, robust, and applicable to virtually any payload protein – precisely as we are all recognizing the potential life-saving power of living medicines, power that can be harnessed broadly by equipping these agents with new capabilities under precise pharmacologic control. Our pharmacologic operating systems are doing that every day in the lab here at 1030 Mass Ave and we can’t wait for the day we can do it in patients whose lives depend on new cures.
Today’s press release is here. And please check out our new website!