This blog was written by Deanna Petersen, Chief Business Officer of AvroBio, as part of the From The Trenches feature of LifeSciVC.
It seems so clear now: gene therapy is on a roll. As 2017 came to a close, the first gene therapy was approved by FDA and more than 2,500 gene therapy clinical trials are ongoing, including over 120 trials in pivotal Phase 2/3 or Phase 3 studies (here). Remarkably, gene therapy is now the second largest class of drugs being developed, bigger than antibodies and only behind more traditional New Chemical Entities (NCEs).
Today, it looks like all blue sky, with a positive climate based on rapid gene therapy progress in recent years. Yet, we know that it took a lot of scientific advances to clear the clouds over the past two decades and enable gene therapy to emerge as one of the most exciting new classes of medicines.
Back to the future with gene therapy 
Twenty five years ago, I also had a seat at the lab bench when we all thought gene therapy was ready for ‘prime time.’ I happened to start my career in the lab of Dr. Mike Welsh at the University of Iowa when he achieved an early milestone with the first AAV gene therapy trial in cystic fibrosis in 1993. The thrill of curing a genetic disease with a single-shot gene therapy seemed so exciting and palpable in that moment. But this program, like many first generation gene therapies, ran into unanticipated roadblocks that kept it from achieving its promise. The deep disappointment of the setbacks that unexpectedly emerged for gene therapy was also intense, and helped to catalyze the transition of my career from academia to the biotech/pharmaceutical industry.
Fast forward to today and I am back in the gene therapy world. The climate has changed and we are ‘back to the future’ with the promise of amazing possibilities with gene therapy. I’m certainly not alone as an entrepreneurial crusader in gene therapy. Biotechs with new ideas for gene therapy technologies are emerging all around us.
What’s interesting to consider is not how the crusaders moved toward gene therapy, but how the stalwarts at large Pharma recognized the early signs that gene therapy had ‘turned the corner,’ so they could make timely decisions for their future plans.
Gene therapy was off of the pharma radar screen for quite some time – a decade or more. In the late 1990’s and early 2000’s, academic institutions were largely responsible for carrying the torch to continue driving the gene therapy field forward. As the academic labs started producing dramatic results, the R&D and strategy leaders at pharmaceutical companies began to take notice.
Since there’s not a time machine to revisit the R&D strategy meetings throughout big pharma to know how gene therapy surfaced back on their radar screens, let me offer a perspective from my experience over the past decade of gene therapy’s re-emergence.
Drawn to biotech for gene therapy innovation
Currently, I am the Chief Business Officer of AVROBIO, a biotech company with a pipeline of lentiviral-based gene therapies. We’ve had recent progress with initial clinical data in October 2017 (as described here as well) for the first gene therapy for the rare disorder Fabry Disease, followed by news today that we secured a Series B financing to advance both our Fabry program and three additional gene therapies for other lysosomal storage disorders.
Before joining AVROBIO, I was no stranger to lysosomal storage disorders (LSDs). For six years through 2015, I was the head of business development and on the leadership team for Shire’s rare disease business, which includes enzyme replacement therapies for LSDs, including Fabry disease, Gaucher disease, and Hunter syndrome. LSDs are rare genetic diseases for which the enzyme replacement therapies have made a remarkable positive difference in the lives of patients.
Enzyme replacement therapies became iconic early success stories in biotech. They can be considered “Biotech 1.0,” the first generation of regularly infused therapies that provide exogenous replacement for a protein that is deficient in patients with these debilitating rare diseases. Great companies like Genzyme (acquired by Sanofi) and Shire/TKT were built through these Biotech 1.0 LSD product innovations.
LSDs are also a perfect target for gene therapy. With gene therapy, the defective gene can be replaced or repaired so that the patient’s own body can consistently produce the crucial enzyme after a single dose therapy. Gene therapy was the obvious Biotech 2.0 for LSDs, and many of us knew it when we were at Shire.
Gene therapy surfaces as Biotech 2.0 in pharma strategy
So, how and why did the R&D leaders at pharmaceutical companies begin to take a new look at gene therapy?
It’s no surprise that the main reason that pharma companies do strategic planning to consider new drug modalities, like gene therapy, is for self-preservation. There’s a survival instinct to anticipate how new classes of therapeutics can enter a pharma company’s established franchise areas. At Shire, enzyme replacement therapies were flagship products in key markets that the company evaluated for the next wave of innovation.
Within the strategy discussions at Shire, there are a host of reasons that emerged for why the barriers to innovation (my twist on the barriers to entry) were lower for gene therapy to be developed for lysosomal storage disorders. Among the reasons that LSDs were recognized as “low hanging fruit” for gene therapy:
- Lysosomal Storage Disorders are monogenic disorders (involve a single gene).
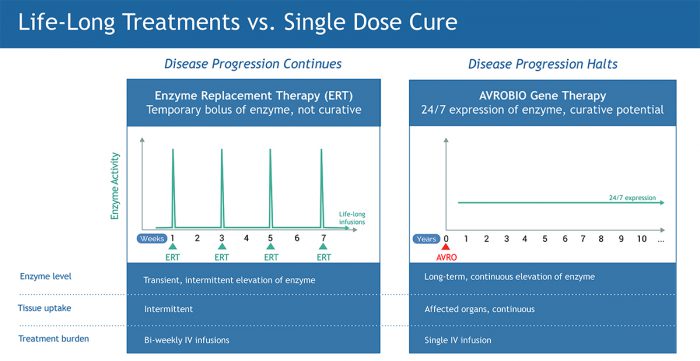
- The therapeutic goal of replacing the activity of an enzyme is easier than for a protein, because an enzyme is catalytic so there is only a need to replace 1-5% of the intracellular enzyme activity in order to have the desired therapeutic effect.
- ERTs proved the concept for the underlying mechanisms of action that gene therapy could also achieve.
- Lentiviral gene therapy is the ideal vector to deliver the genes involved in LSDs, and lentiviral technology had been achieving successes in various gene therapy applications for safe integration and sustained duration.
I recall many strategy discussions at Shire about how to equip the company for a scenario where gene therapy would become the future paradigm for lysosomal storage disorders, potentially supplanting ERTs. It was the typical innovator’s dilemma – how can market leaders avoid the common failure to seize the next wave of innovation in their industries?
At Shire, I worked closely with Arthur Tzianabos who headed research and early development, and we were both convinced that gene therapy was on a fast track. But, Shire was like many larger pharmas. It’s not trivial for them to get their arms around a gene therapy strategy. They rightfully view gene therapy as a complex drug modality that isn’t quick or easy to say ‘yes’ to in the near term. And, of course, mature businesses put their attention on other pressing business priorities.
Arthur and I both ended up working at gene therapy biotechs. We ultimately expressed our enthusiasm for gene therapy by voting with our feet. He’s now the CEO of Homology, focusing on the same sorts of business-building activities for gene therapy that I’ve spent the past two years doing at AVROBIO: advancing technology, rolling up the know-how and assets, and implementing a fast and nimble biotech strategy.
Shifting a paradigm, following in the footsteps of others
Indeed, with gene therapies we are shifting the paradigm. But, as we step out into new domains, we are also encouraged to be following pathways that have been trodden by other drug development pioneers.
First, technology achievements have been attained. In the realm of lentiviral gene therapy technology, GSK and Bluebird Bio have proven the power of lentivirus in a range of disease areas.
Second, market landscapes have been established. For gene therapy applications – such as hemophilia and lysosomal storage disorders – the gene therapy is aiming to supersede protein replacement therapies that are already approved and widely used. While it is always challenging to set a higher bar when existing treatments are in place, there are also advantages from following in the footsteps of an approved ‘replacement’ therapy. Existing drugs provide an established pathway for clinical development and clinical endpoints, increasing the probability of success and avoiding the need for natural history studies. Existing drugs also pave the way for adopting and reimbursing a new treatment for the disease within the healthcare community.
At AVROBIO, we aspire to put our expertise, ingenuity and resources to work so we can help to further advance the gene therapy field, specifically in our core area of lentiviral technology and in our initial focus for advancing potential cures to patients with lysosomal storage disorders.
The big picture and coming full circle
We are in a time when the path of innovation in the drug industry has hit a precipitous pace. The impact that gene therapy can have for patients is profound: a single-dose therapy can offer a new model for living a normal life. For patients with lysosomal storage disorders, gene therapy would supersede the bi-weekly infusions of enzyme replacement therapies that they receive over their lifetime, which do not prevent their disease from progressing. As a new paradigm, gene therapy offers hope for a single dose cure.
The exhilaration I felt with gene therapy 25 years ago in Dr. Welsh’s lab never left me. But it’s even more exciting now that these transformative therapies are achieving their promise and decisively making their way to patients.
The time has arrived.