The field of gene and cell therapy has witnessed an unprecedented few months, almost unimaginable only a few years ago. It’s worth pausing to reflect on some of the recent highlights:
- FDA approval of two chimeric antigen receptor (CAR) T-cell therapy products, Kymriah from Novartis and Yescarta from Kite/Gilead, that have repeatedly shown profound efficacy
- Positive unanimous recommendation from a group of FDA advisors (and patients) for Luxturna, an adeno-associated virus (AAV2) gene therapy from Spark Therapeutics, for a rare form of blindness, delivered via subretinal injection
- Continued positive results from ex vivo lentiviral therapy in cerebral adrenoleukodystrophy (CALD) from bluebird bio’s STARBEAM trial, published last month in the New England Journal of Medicine
- Striking improvements on survival and motor function from a single dose of AveXis’ AAV9 encoding SMA1 in children with spinal muscular atrophy, according to another NEJM paper published this month
These are indeed exciting times – and the pace of innovation seems incredible. Living medicines based on cell and gene therapy have moved into mainstream on the promise of compelling clinical data, and there are literally dozens of companies, big and small alike, advancing the next generation of this genre of therapies. It’s worth pausing to reflect on how far and fast we’ve come.
An Overnight Success
A recently as 2010, few could have dreamed of this kind of progress so quickly in the cell and gene therapy (CGT) field.
Gene therapy was still largely in the penalty box at that time. Systemic AAV approaches ground to a halt after the death of Jesse Gelsinger, who died during a gene therapy trial for ornithine transcarbamylase deficiency (OTCD) at Penn in 1999. Most private sector companies who dabbled in the field earlier had left it, leaving only a few brave academic investigators to keep the field moving.
Shortly after adenoviral vectors had their safety crisis, ex vivo retroviral therapy hit concerns, too: reports emerged of acute leukemia amongst the first patients with “bubble boy” disease (a type of severe combined immunodeficiency, SCID), who had received bone marrow transplants with retrovirally-transduced cells. These cancers were likely due to apparent oncogenic integration events caused by the retrovirus, when it inserts itself into a bad location in the genome and causes cancer. This raised the red flag for ex vivo retroviral therapies, slowing broader investment and interest in the field for much of the 2000s.
Similarly, CAR-Ts had been around for nearly twenty years by that time with little compelling clinical data: initial constructs just lacked the right configuration to deliver robust in vivo stimulation and expansion, a requirement for strong anti-cancer effects.
But hints that things would change emerged 7-10 years ago.
Ocular delivery of AAV2 in rare orphan blindness began showing early efficacy in several clinical trials in 2008 (here, here, here). Ex vivo retroviral therapy for ADA-SCID appeared curative, with better safety margins, in a small ten patient trial in a landmark 2009 NEJM paper, as well as lentiviral delivery in a pair of ALD patients as published in Science later that year. And early reports that next generation CAR-T constructs may be working appeared in 2010, culminating with a NEJM paper in August 2011 as a case report of a CLL patient treated with CD19-CART at Penn with a dramatic response. Also, in that same month in 2011, MSK investigators published data on their clinical trial of ten patients with ALL in Blood.
All of these very small “n” studies began to change the momentum of the CGT field – engendering the early optimism which fueled both risk-taking and initial funding to extend the progress, and begin the movement from academia into industry (the “outside-in technology evolution). And that excitement then allowed the investment floodgates to burst open just a few years ago.
With that backdrop in mind, it’s worth reflecting on the pace of innovation since those small studies captured the world’s attention: the CART field went from reporting the first truly efficacious patient data to a pair of FDA approvals in just six years. That’s a quick clinical cycle time for any drug candidate, even more so for a totally novel, living medicine with unprecedented cell handling and processing protocols.
Yet, at the same time that, while this pace seems fast on some measures, over the arc of biotech history it’s also characteristically slow in others. The CGT field is clearly standing on the shoulders of giants and benefiting from decades of groundwork, as Magenta CEO Jason Gardner wrote in a blogpost a year ago. The early SCID transplant work started around 1992, and for almost two decades remained an academic quest to prove out the technology, but eventually resulted in the first ex vivo retroviral cell therapy approval in the world, Strimvelis for ADA-SCID, in spring 2016. The first CAR-T concept was described by Zelig Eshhar in 1989 in PNAS, nearly 30-years before the first approvals this year.
CGT is clearly an overnight success that has taken a few decades.
Power Of Small N’s With Compelling Data
One of the salient takeaways from the history of the CGT field is the catalytic impact of truly compelling early clinical data. An axiom in drug R&D is that “great drugs reveal themselves early” and this is never more true than in the CGT space. One doesn’t need to “torture the data” to make it yield answer. In many instances the answer is clear within a few patients. With such profound unmet needs in most of the grievous, debilitating conditions where these CGT approaches are appropriate, when CGTs work well, one can often see it quickly and with very small numbers (n’s) of patients. And that holds true even in later stages of development: take Luxturna’s Phase 3 study, which only has 21 patients; Strimvelis was approved with 18 patients.
This is frequently because of the precise link between the pathophysiology and the gene therapy’s action: replace the lost gene with endogenous continual expression. Early exogenous enzyme replacement breakthroughs highlighted this, too. Or beyond genetic diseases, engineering cells like CAR-Ts to co-opting the powerful, multifactorial pharmacology of the human immune system to attack specific antigens on cancer cells.
Given the complexity of these therapies, profound early efficacy, or robust confidence from biomarker data, is almost essential for CGT enthusiasm.
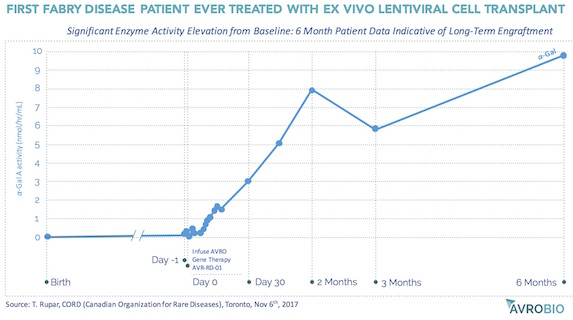
AvroBio, an emerging ex vivo lentiviral CGT company, is a good example of this. Founded in 2015, and seeded by and incubated at Atlas Venture, the vision of AvroBio is to treat lysosomal storage diseases with curative intent through ex vivo lentiviral stem cell transplants. AvroBio builds off of nearly twenty years of preclinical work from the labs of Jeff Medin and Roscoe Brady (the latter being the discoverer of the genetic defects of several LSDs), Stefan Karlsson at Lund, and Stephanie Cherqui at UCSD. Based on preclinical work in Fabry mouse models, and in collaboration with the Canadian multi-center FACTs group, the first-ever ex vivo lentiviral transplant therapy clinical trial to treat Fabry’s Disease was recently initiated involving minimal intensity “outpatient” conditioning. Without hyperbole, the initial clinical data – yes, an n=1 – is the most compelling initial signal I’ve ever seen in a trial: as shown in the chart below, shared at the recent Canadian Organization of Rare Disease (CORD) meeting, six months after the transplant with lenti-transduced autologous CD34+ cells, the first patient’s Alpha-Galactosidase A enzyme activity levels are now well into the normal range, after living his entire life without endogenously-expressed enzyme. Assuming enzyme activity levels correlate with long-term outcomes, which seems reasonable, this patient may be cured. Exciting times for the Fabry patient community, and for AvroBio as it advances its portfolio of ex vivo lentiviral CGT for Fabry, Gaucher, Cystinosis, and Pompe diseases with curative intent. Three of those will be in clinical testing by this time next year.
Regulators Becoming Compelled By Transformative Data, Too
It’s not just investors who get excited by high impact early clinical data in the CGT field. Regulators have begun to appreciate the power of these therapies, especially in rare genetic diseases.
In May 2017, at the Food and Drug Law Institute’s annual conference, CBER director Peter Marks highlighted the impact of the 21st Century Cures Act in bringing new tools to bear, like the Regenerative Medicine Advanced Therapy (RMAT) designation. When final, the RMAT process will help expedite new CGT clinical programs to FDA approval.
Further, Marks went on and said that “single arm, compelling studies” are likely all that will be needed, saying “…trials of 10 or 20 patients for gene therapy trials for rare diseases may be compelling enough for approval…”
Considering how novel and unprecedented CGT products are – virus/cell manufacturing and handling, often with transplant procedures – getting the FDA and other regulators engaged in guidance like the RMAT will be hugely facilitating of the field. These aren’t your grandma’s pillbox therapies; new approaches will be needed to both clinical and regulatory standards.
Looking Forward to ASH17
The upcoming Dec 2017 American Society of Hematology meeting promises to highlight further advances in the CGT field, and reinforce how “mainstream” these living medicines have become. The #ASH17 abstracts were unveiled last week and provide lots of substrate for enthusiasm.
On CAR-T front, plenty of excellent engineered T-cell therapy progress in a range of indications. BCMA CAR-Ts from bluebird and Novartis continue to deliver robust overall responses rates (ORRs), in 50-60 combined patients (as of abstract submission dates). Updated DLBCL data from multiple CAR-T players like Juno, Novartis, and Kite/Gilead continue to impress with high ORRs. A broad range of next generation approaches like switchable CAR-Ts (e.g., here, here) and allogenic CAR-Ts, as well as Unum Therapeutics’s ongoing refractory NHL trial (ATTCK-20-2) with its Antibody Coupled T-cell Receptor engineered T-cells in combination with Rituxan.
In the lentiviral CGT space, we’re seeing including real improvements in vectorology (e.g., better promoters, constructs), more efficient virus and cell manufacturing, higher (and higher quality) cell doses across a range of conditions – all leading to better transplant chimerism in patients (i.e., higher “vector copy numbers”), reflecting a greater presence of cells in the hematopoetic lineage with the corrected genetic material. Both bluebird (LentiGlobin) and the San Raffaele Telethon Institute for Gene Therapy (SR-TIGET, with their GLOBE LV) are presenting data in blood disorders like thalassemia. The elevated vector copy numbers (VCNs) that bluebird is seeing in the new sickle cell disease patients suggest improved manufacturing and other measures may be having real impact. In addition, SR-TIGIT will highlight data using a novel intraosseous delivery route (injected transplanted cells directly into the bone to engender higher engraftment rates, higher VCNs). Another program from SR-TIGIT, in collaboration with Bioverativ, is highlighting it’s liver-directed in vivo “immune stealth” lentiviral approach for hemophilia B (here). Lastly, and related to cell transplant therapy more generally, Magenta Therapeutics is sharing several oral and poster presentations on CD34+ HSC expansion, as well as targeted non-genotoxic conditioning regimens – both broadly important areas as the CGT field advances.
In a very much deserved acknowledgement for their contributions to the field, Luigi Naldini and Marina Cavazzana, two pioneers in lentiviral therapy, are being honored with the Ernest Beutler Lecture (here). Further evidence that CGT field has moved into the mainstream, an overnight success on the shoulders of giants.
Disclosure: Atlas Venture is an investor in AvroBio, Unum, and Magenta, all mentioned in the post.