By Ram Aiyar, CEO of Korro Bio, as part of the From The Trenches feature of LifeSciVC
It has been an extraordinary journey so far and one that continues to humble, inspire and motivate me every day. I’ve had the honor and responsibility of building a visi on that is ambitious while being grounded in scientific rigor. Over the last 4.5 years, Korro has been built by mitigating one layer of risk at a time. This has culminated in a strong team, a robust pipeline, a set of supportive investors, board members, and key opinion leaders.
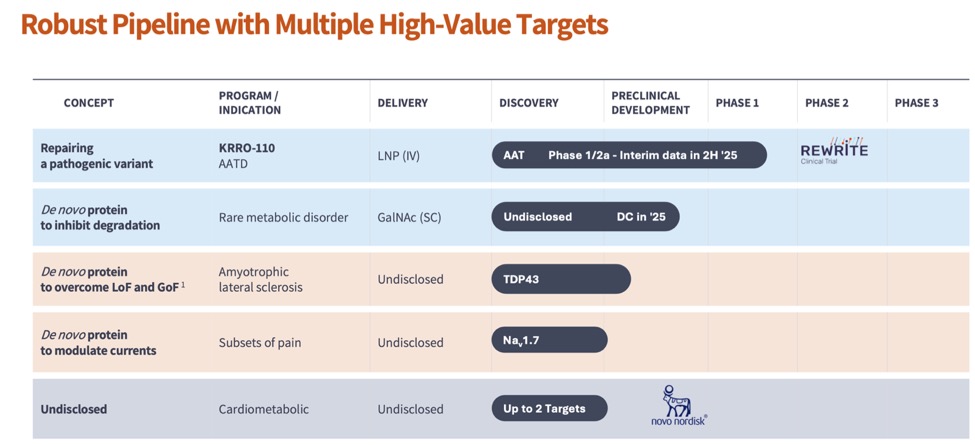
2025 holds to be a transformative year for us, with the potential for multiple milestones to come to fruition, with the biggest value inflection being the interim clinical data from our lead asset KRRO-110, a potential best in class compound for patients with Alpha-1 antitrypsin deficiency (AATD). To learn more about Korro Bio see here.
Founding Vision: Harnessing RNA Editing for Transformative Medicine
Korro Bio’s story began in 2018, co-founded by a remarkable group: Jean-François Formela, M.D. (Partner at Atlas) Nessan Bermingham Ph.D. (Founder and Ex-CEO of Intellia), Andrew Fraley, Ph.D. (Scientist entrepreneur), and Josh Rosenthal, Ph.D. (Academic specializing in RNA editing). Their collective expertise—spanning gene editing, venture creation, and foundational science—set the stage for building Korro. Josh’s work at the Marine Biological Laboratory unlocked the potential of utilizing an endogenous protein to make a single alphabet change on RNA (RNA editing), a process that was precise, reversible and had druglike characteristics. The ability of modifying RNA enabled us to sidestep many of the potential risks associated with permanent DNA editing. Andrew and Ness with the operational experience building companies, pulled together the foundation and demonstrated with preliminary data the concept of using an oligonucleotide to enable RNA editing. Atlas Venture and NEA were early believers, incubating Korro Bio and providing the initial capital and strategic support needed to turn this vision into reality.
Building medicines by activating biological pathways
As I joined the Company in Q4 2020 as its CEO, I had a vision of creating protein variants to activate biological pathways. This ability to activate pathways could be possible for almost all proteins, however the biggest differentiation was to consider areas that were hard to drug – transcription factors, ion channels, intracellular proteins, etc. This concept of effecting a protein change. was based on experience building on the understanding that a single protein variant, could have markedly different outcomes for patients with chronic kidney disease.
From the outset, our mission was clear: to discover, develop, and commercialize a new class of therapies that could impact a broad range of diseases, both rare and prevalent. Our goal was to do provide a paradigm of activating biological pathways in a highly targeted manner. We aimed to achieve this by modifying and modulating proteins by precisely changing a single alphabet on RNA with the use of an oligonucleotide (think short stretches of chemically modified RNA or DNA). We intended to learn from nature (genetics) and use pharmaceuticals properties to drive patient benefit. We built our proprietary OPERA™ platform (here), leveraging oligonucleotide guided RNA editing to modulate protein expression.
We could not get into prevalent diseases from the outset, as taking on the risk of novel technology in a large indication compounded the risk increasing risk of failure. If something were to go wrong along the way, we would have taken too much capital and would not have known why we failed. As I wore my engineering hat, the most prudent way was to modify one variable at a time, and control known variables.
Removing Risk, Each step of the Way, Step by Step
How does one work on novel science, continue to have confidence in the path taken and generate sufficient evidence to create value and therefore enable a financing? Every biotech journey is a lesson in humility, especially when working on novel mechanisms and novel science.
One of the first important decisions was to choose an indication. Alpha-1 Antitrypsin deficiency was selected based on unmet need and scientific rationale (video).
- Big unmet medical need – Needing to solve two problems at a time
- too much bad protein in the liver, not enough good protein in the lung
- By repairing the protein in the liver, we could simultaneously solve two biological problems with one approach
- Multiple ways to deliver the drug to the liver with the precedence of approved drug products
- Ability to know if the mechanism works patients in the first clinical study as the repaired protein would go from non-existent to high levels
- We will likely know if we have a drug in the first study in less than 30 patients
To ensure we would work in this setting early, we ran the first experiment with a fully synthetic oligonucleotide encapsulated in a Lipid Nano Particle (LNP) in the gold standard PiZZ mouse model for AATD showing low double digit editing with high specificity and correlating protein expression. The experiment worked and showed the precision of RNA editing.
It was a time where “platforms” were in vogue, talent jumped shipped often, people working from home during COVID and all the while working towards developing a clinical candidate. It was hard, and I was not entirely successful, but changing the mindset of much of the company from a technology focused company to a drug development entity required focus.
Next, capital allocation and company build rested on the question of whether the concept of making an amino acid change in protein was relevant biologically and how we would generate that evidence. We assembled a group of consultants, key opinion leaders, experienced drug hunters, and charged them with the question:
- Find a known target where biology was well known, but traditionally undruggable and identify points of differentiation in specific diseases
- In addition, demonstrate feasibility with 3 – 6 months with external resources
Within 6 months, this motley crew of non-FTEs, was able to generate preliminary evidence that the concept of changing an amino acid to activate biological pathways is possible, and repeatable across multiple targets. It also built credibility to the fact that we were not focused on Mendelian disease to ONLY repair a protein that was mutated due to a defect on the DNA, but rather learning from genetics and modifying an existing protein that is functional. Knowing that we can go after multiple targets, we set out to iterate on the compounds to create increasingly potent molecules. The investment into building a “platform” that could generate multiple assets, was starting to become real. This process has led to us building a robust pipeline.
Knowing that the siRNA and ASO gapmer field (here) went through iterations in potency and delivery, with a novel mechanism we wanted to ensure success with the first compounds in the clinic. This meant focusing on clinical benefit for patients with AATD and build a drug candidate profile that was meaningful for patients.
What is the clinical need:
- AATD patients needed to get to at least heterozygous levels of protein (~50% of all RNA transcripts modified leading to “normal” protein)
- infrequent dosing
- Generate evidence that this mechanism and specifically this drug candidate will work in humans
Risks:
- If activity is not demonstrated in humans, is it because the drug candidate did not get to the right place in the cell (cytoplasm or nucleus) OR is the mechanism not working?
- Is it more important to have a clinical meaningful drug candidate OR convenience? Standard of care for patients with AATD is once-a- week infusion.
We thus picked an LNP to move forward, to ensure drug was in the right compartment at high concentrations and not stuck inside an ineffective compartment. We removed a layer of risk by picking an LNP after running experiment with multiple vendors which had the right profile for us (high animal safety Index, and evidence that the LNP had been in humans with a good profile). Additionally, we confirmed using tool compounds that the mechanism has a high probability of working in humans (translational studies in mice and monkeys). Finally, we only nominated the development candidate KRRO-110 after running several safety studies in rodents and monkeys. We were able to do this by generating another round of private financing in Series B and finally taking the “not-frequently-travelled-path” of undergoing a merger, a public listing and a private financing ALL at the same time (reverse merger). This meant keeping a focus on the scientific team on the ambitious goals, focusing the finance and legal team on the multiple transactions, educating the investor and analyst community on the potential of our data, and navigating the ever-evolving competitive landscape.
Generating clinical data in patients is one of the most meaningful value inflection points. We would not be here were it not for an excellent board, scientific advisors, and a team focused on execution AND individual sacrifices to accomplish the impossible. It has never been easy, we have not always had all the resources we needed, and I am extremely proud and grateful for the team to “Say what we are going to do and Doing what we said we would”. We have stuck to our timelines that we laid out in ’22.
But great science means little without great people that we have had the pleasure to being at Korro – scientists, clinicians, operators, and culture-builders who bring both expertise and heart. Thankful for each of the individuals that have contributed to getting us to where we are today.
What I have learned putting all of it together (outside of GPT) and with my other 6 companies
- Have a bold vision that is uniquely yours (and aligned with the BoD)
- Plan for 5 – 10 years ahead with building the market-product fit for each asset
- Build the company to increase PoS (taking layers of risk off at a time) with each financing
- Decide early before capital formation, are you a single asset company or more
- Meaningful clinical data is the single most value creating event for a biotech (try to get it done in the first trial)
- There are easier ways to make money than being in a Biotech – make sure you are in it for the patients (anything else will pale in comparison)
- People in the end are what matter through the journey (colleagues, BoD members, investors, KOLs, SAB members, competitors and most importantly the patients)
The Road Ahead: Grandeur with Humility
Our ambition remains as grand as ever—to rewrite the future of medicine, one RNA edit at a time. We have laid out an ambitious 3-2-1 strategy to get three clinical assets, across two tissue types with a single platform through 2027. We approach this mission with humility, knowing that each step forward is earned through teamwork, learning, an unwavering focus on patients and generating evidence along the way.
These are exciting times for us at Korro, and the field in general! Despite the external market environment, as long as we generate hope for patients and provide an option to transform their lives, everyone at Korro is excited on the possibilities of RNA editing and the potential benefit it can bring for patients.
To everyone who has joined, invested in, advised, or supported Korro Bio: thank you for believing in this vision and for driving it forward with passion and integrity. The journey is far from over, and I’m honored to continue building this organization alongside you.
Certain statements in this blog may constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements include, but are not limited to, express or implied statements regarding expectations, hopes, beliefs, intentions or strategies of Korro regarding the future including, without limitation, express or implied statements regarding execution of the 3-2-1 strategy, among others. Forward-looking statements are based on current expectations and assumptions that, while considered reasonable are inherently uncertain. Nothing herein should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved.