By Gerry Harriman, CSO and co-founder of HotSpot Therapeutics, as part of the From The Trenches feature of LifeSciVC
As scientists, we are accustomed to learning something new about targets (and the modulation thereof) that moves the field forward in some way. In reality, such a learning is often incremental and while important, may not be ground-breaking. In rare opportunities (potentially once in a lifetime as a CSO!), there is the potential to unearth a completely new finding for a target that completely alters the paradigm for a disease.
Within the solid tumor landscape, perhaps no target better exemplifies the complexity of the arms race between mutational oncogenic drivers and targeted therapeutic interventions than those tumors associated with the target KRAS, one of the most commonly mutated RAS genes in cancer. Starting in the 2010s, the discovery and development of the first KRAS inhibitors has been revolutionary.
This has led to real clinical success and conferred critical benefit to patients, including in diseases like non-small cell lung cancer (NSCLC), and with new therapeutics in development, such as Revolution Medicines’ exciting pan-KRAS inhibitor RMC-6232, promise now exists for patients with other tumor types, including pancreatic cancer. But as often the case in the war against cancer, the enthusiasm around these data comes with caveats: many patients are de-novo resistant, and the vast majority develop resistance.
Colorectal cancer (CRC) is one tumor type that remains stubbornly resistant to novel targeted therapies. CRC tumorigenesis is driven by multiple successive mutations that activate both pro-survival and proliferation pathways. KRAS inhibitors have a more limited impact on pro-survival pathways, which may explain the low clinical response rates observed in CRC versus other tumor types, as shown in the figure below. As slowly growing cells can accumulate new mutations that enable them to evade therapy, an approach that not only impacts proliferation, but also drives tumor cell death (apoptosis), may more effectively attack cancer in this tumor type.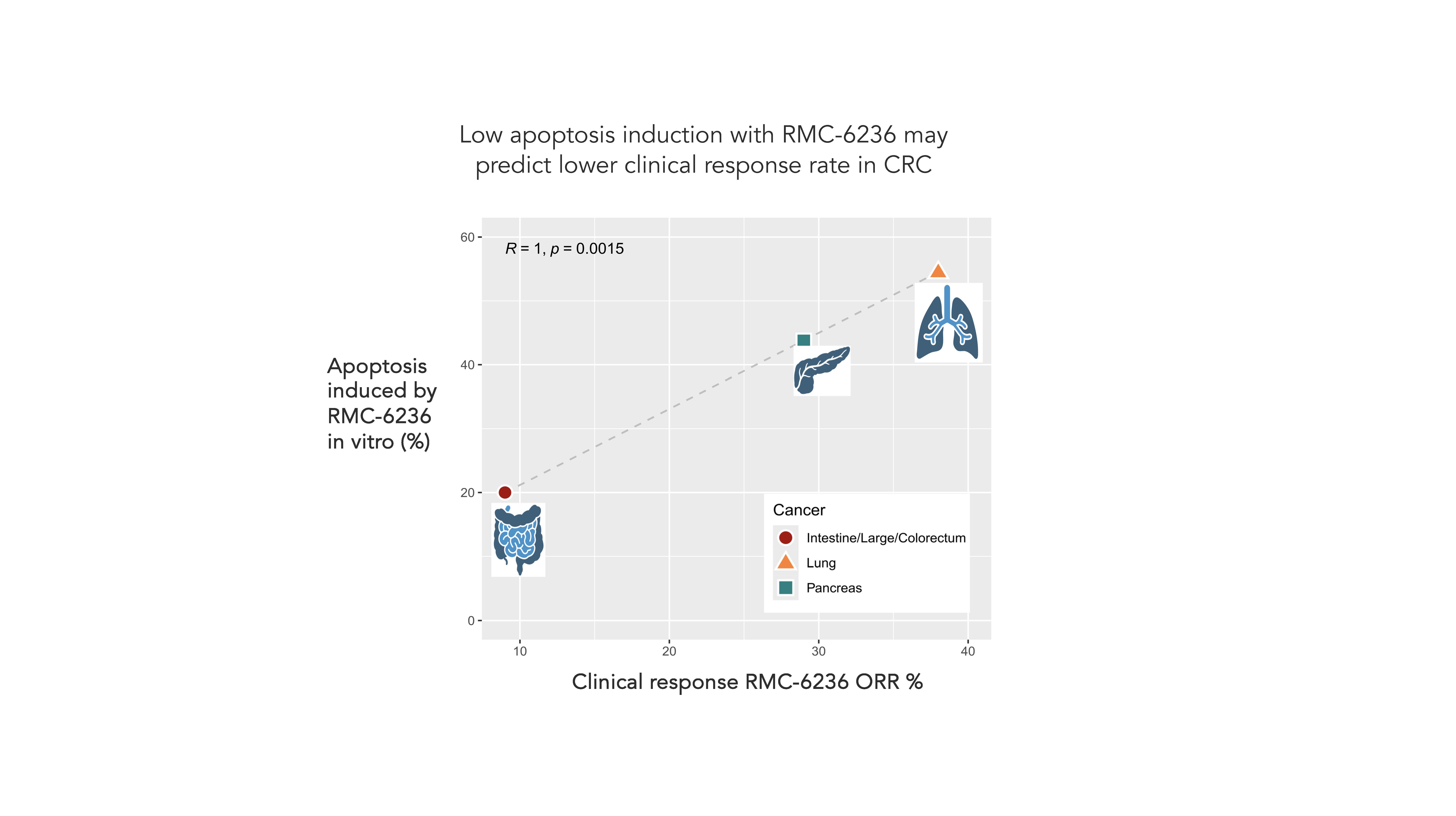 Furthermore, KRAS G12V and G12D mutations are most common in CRC, which align poorly with the currently approved KRAS inhibitors that inhibit only G12C. Unfortunately, these dynamics largely play out clinically, where response rates and durability of KRAS inhibitors lag substantially behind those of other tumor types.
Furthermore, KRAS G12V and G12D mutations are most common in CRC, which align poorly with the currently approved KRAS inhibitors that inhibit only G12C. Unfortunately, these dynamics largely play out clinically, where response rates and durability of KRAS inhibitors lag substantially behind those of other tumor types.
At HotSpot we have sought to address these two challenges through a novel approach to CRC that (i) inhibits both pro-survival and proliferation pathways and (ii) has the potential to inhibit a broad swath of KRAS G12X mutations.
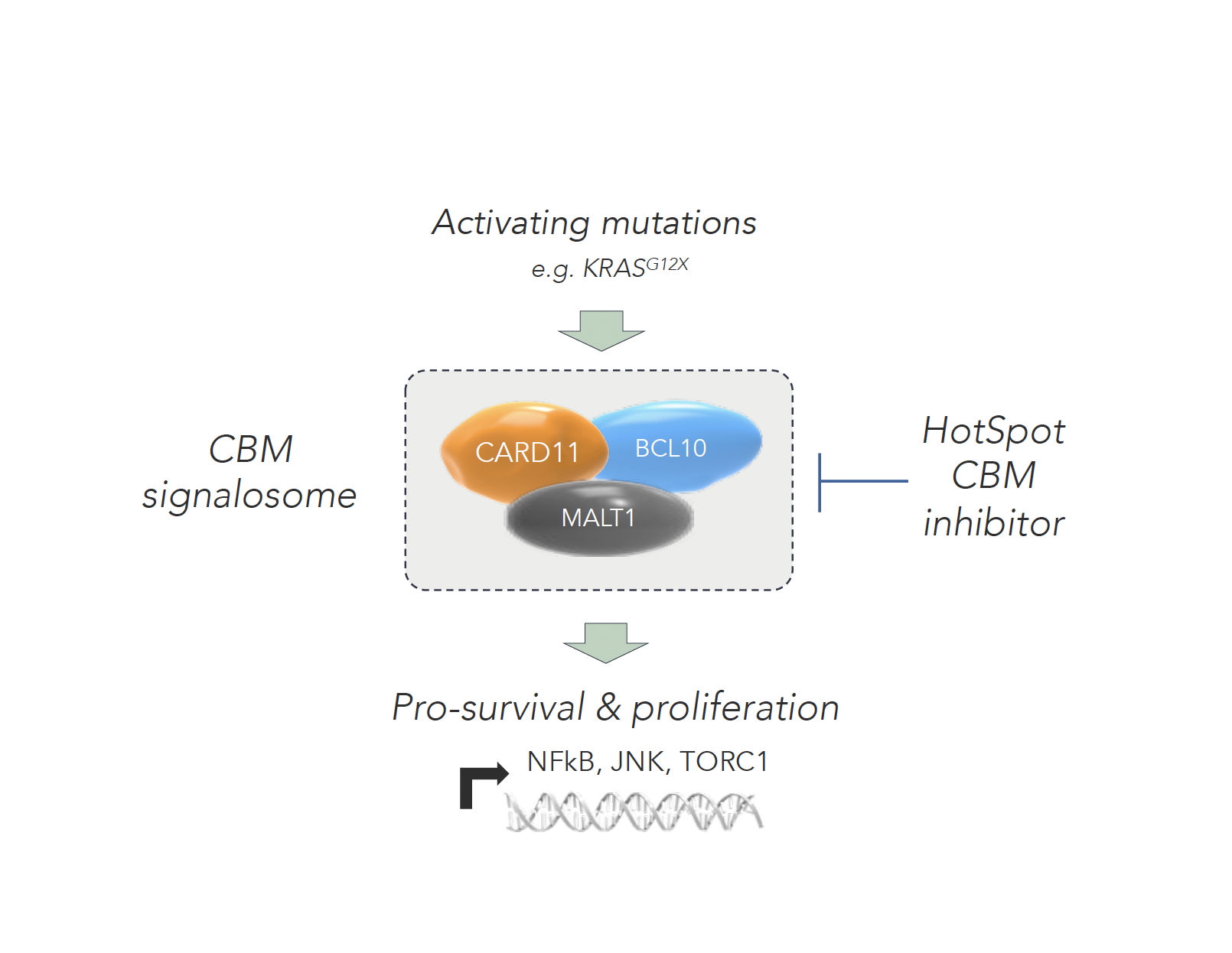
Not surprisingly, it’s easier said than done – but at HotSpot, we’re incredibly excited to share that we have discovered a new synthetic lethal relationship between KRAS G12X and the CBM signalosome complex.
The CBM signalosome is made up of three key proteins (CARD11, BCL10 and MALT1) and regulates key pro-survival and proliferation pathways, including NFkB, JNK, mTORC1 and MYC. By controlling these multiple key pathways, the inhibition of the complex itself presents an opportunity to impact the multiple drivers or contributors of both cancer cell survival and proliferation that are often mutationally driven.
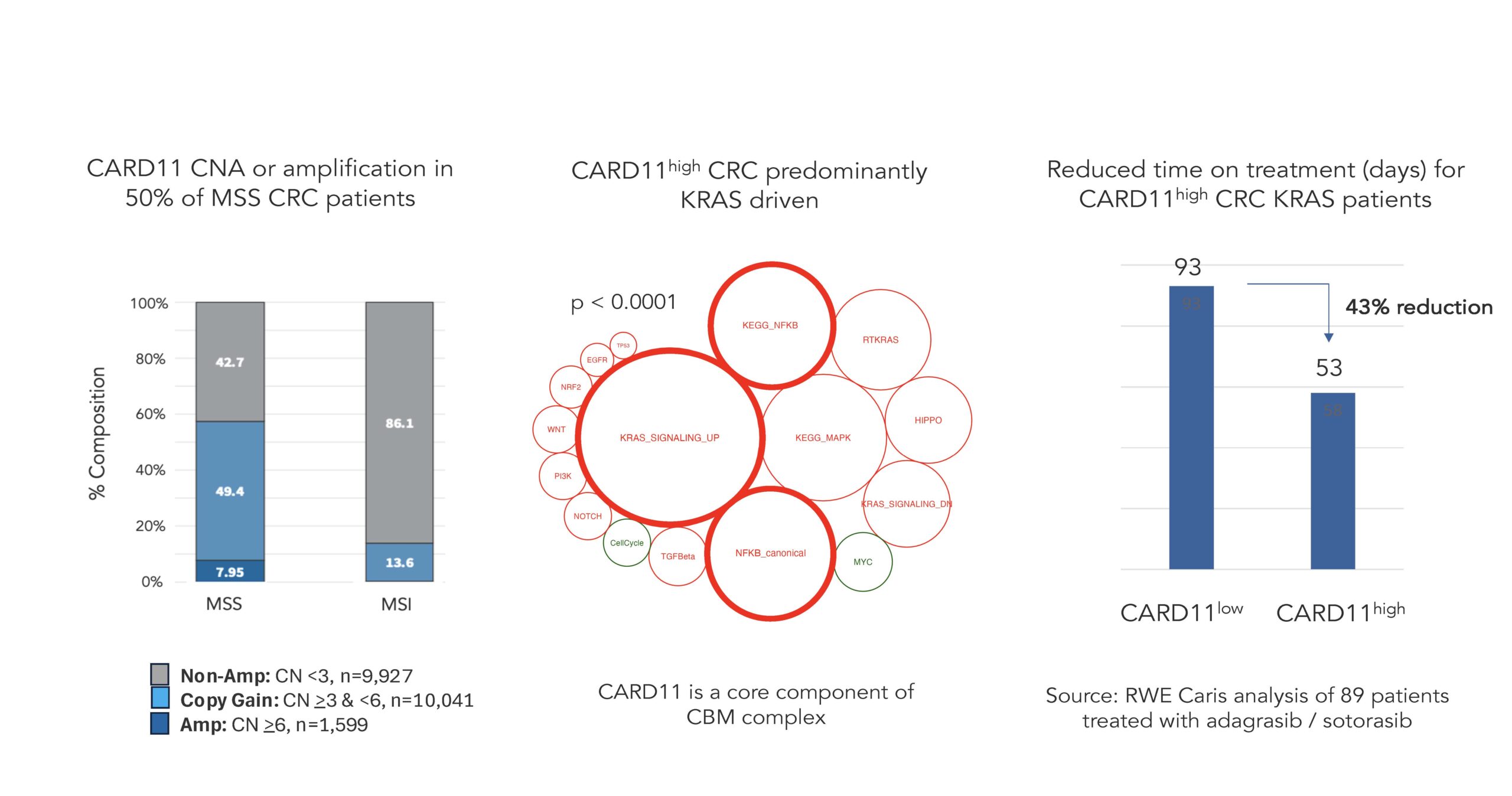
To generate the novel data that conclusively demonstrate the linkage between KRAS G12X and the CBM signalosome, we leveraged our proprietary collaboration with Caris Life Sciences to examine the role of the CBM complex in real world patient data where CARD11, a key component of the CBM complex, is a hallmark of CBM-driven colorectal cancer, as shown in the figures below.
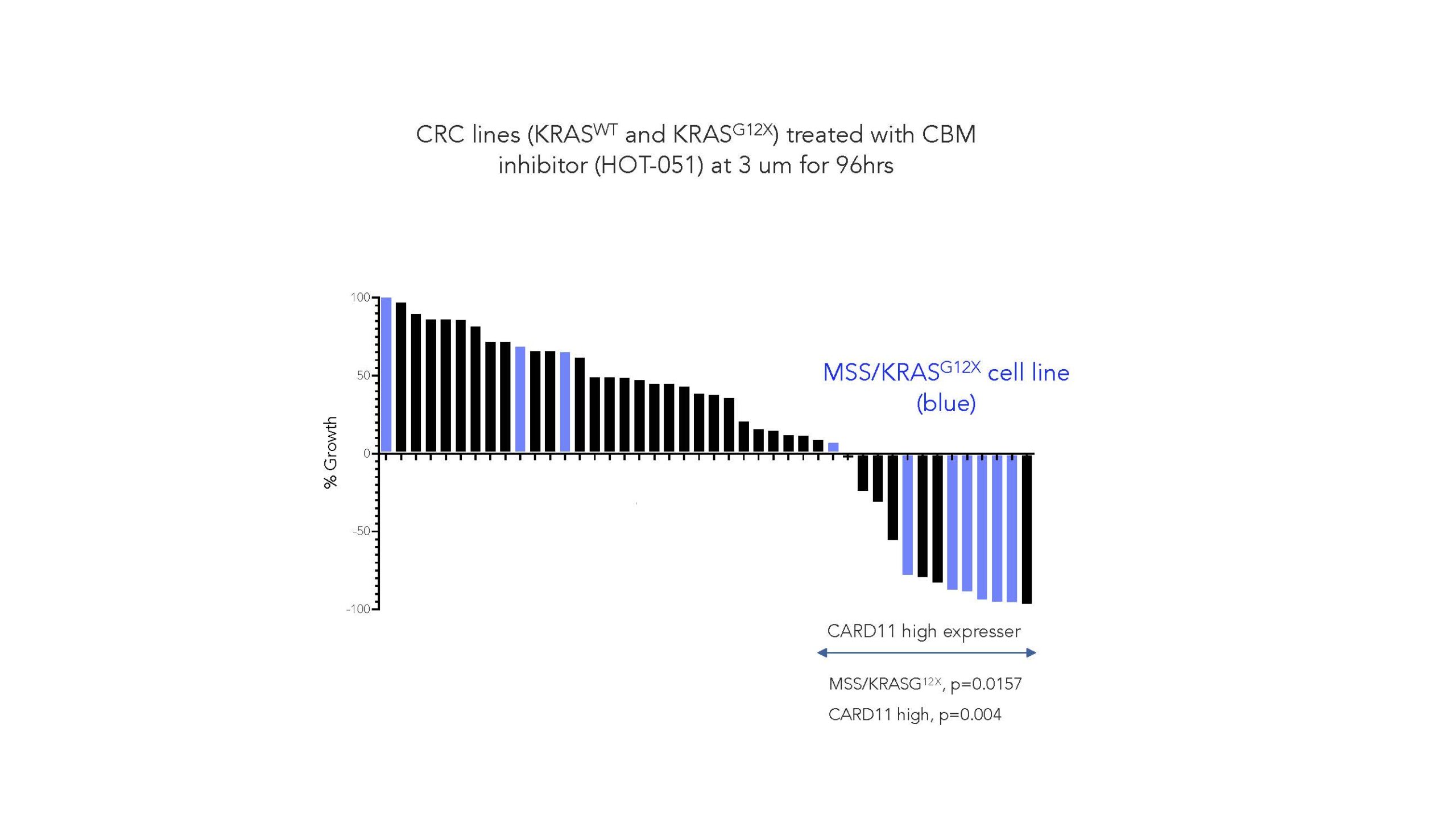
Harnessing our Smart Allostery™ platform, we have developed the first small molecule inhibitors of the CBM complex to demonstrate binding the signalosome and locking it into an inactive conformation. Critically, we have demonstrated that a CBM inhibitor selectively induced apoptosis and cell death in MSS/KRASG12X tumors (see figure below, in blue).
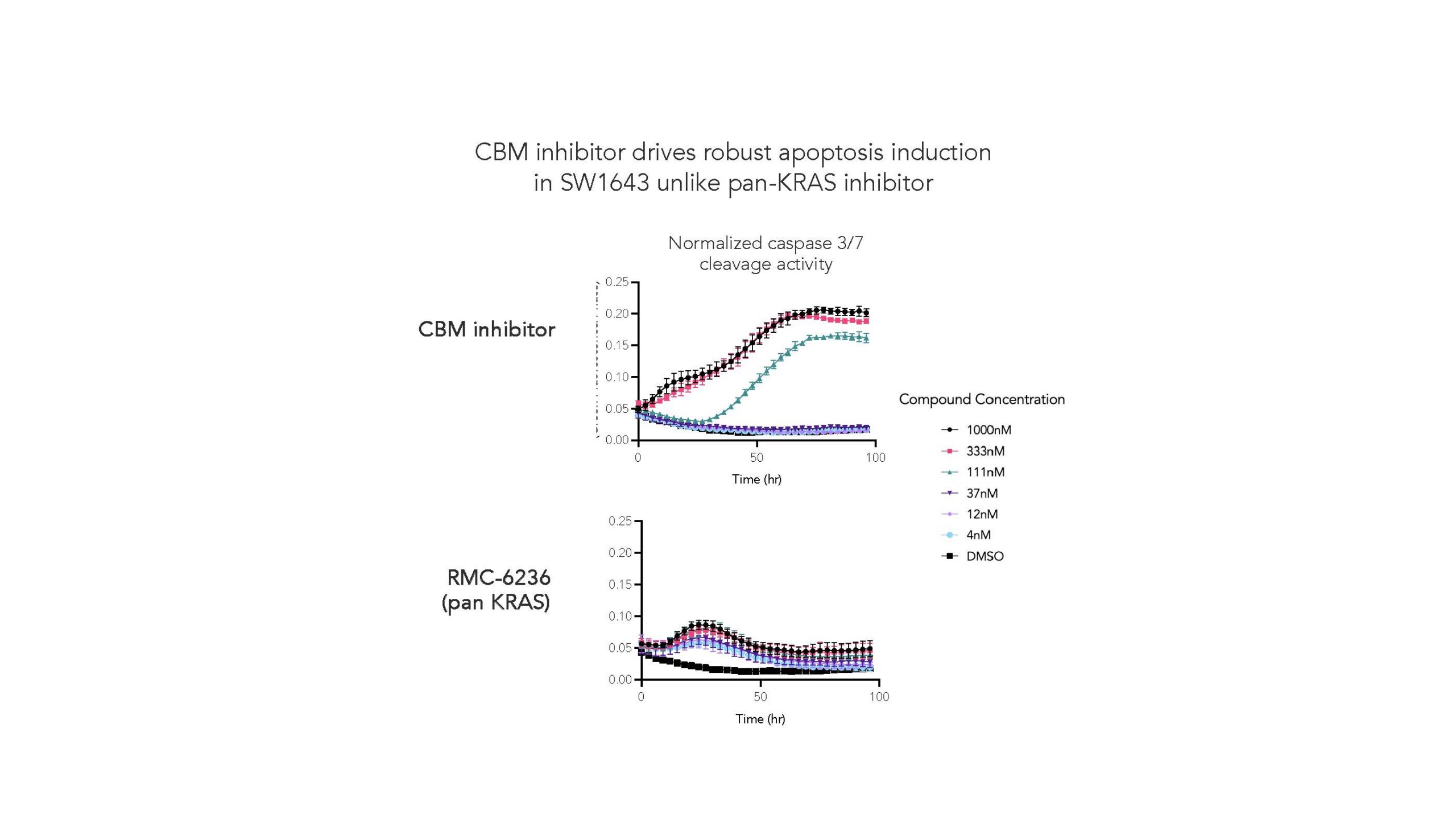
Examination of numerous KRASG12X cell lines, including the SW1643 cell line, demonstrates that CBM inhibitor drives robust apoptosis in comparison to the leading pan-KRAS inhibitor RMC-6236.
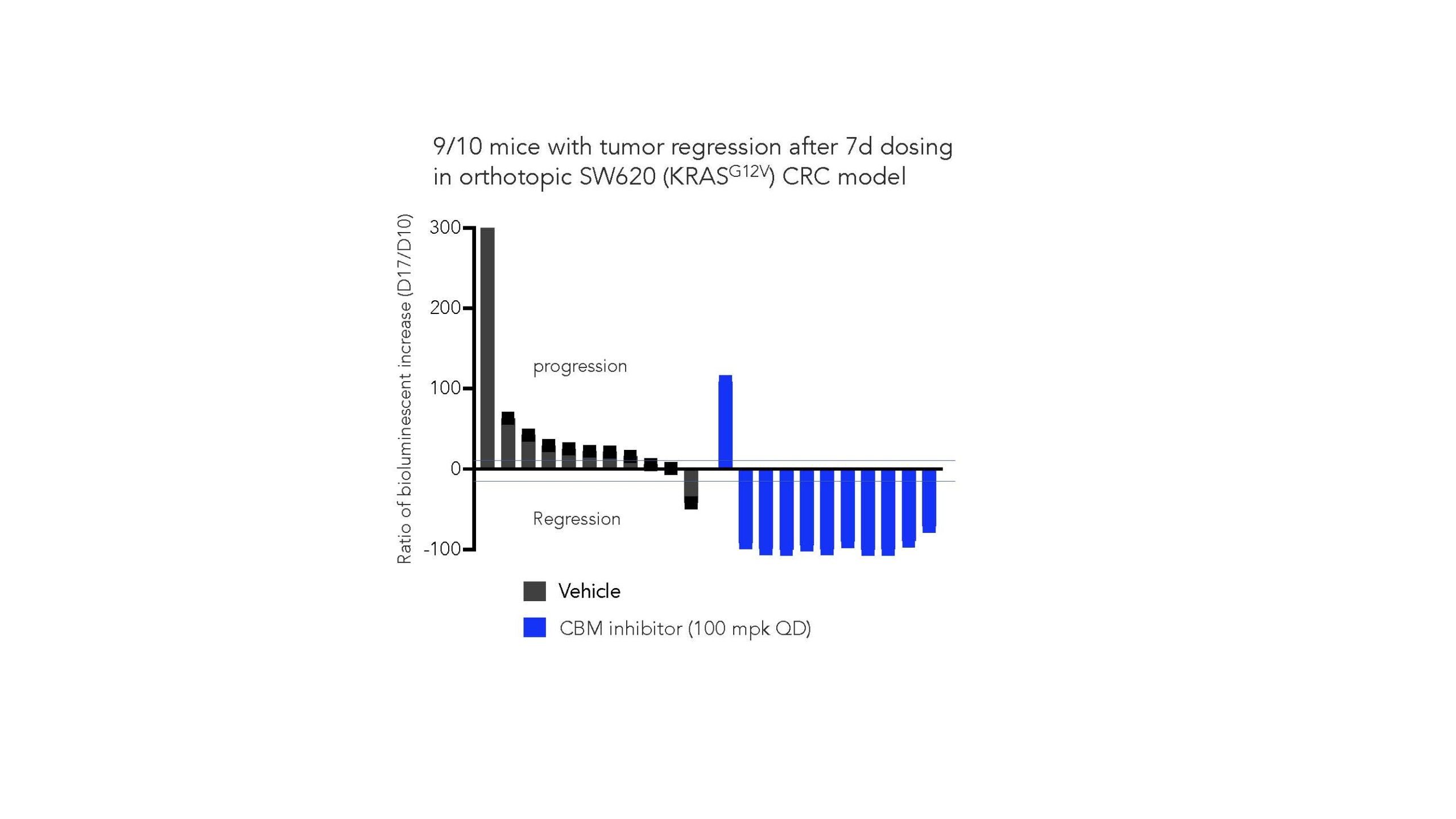
As we move into an in vivo setting, we further demonstrate that CBM inhibition can drive regression in an orthotopic model with 9/10 mice experiencing close to 100% tumor regression, as shown in the next figure:
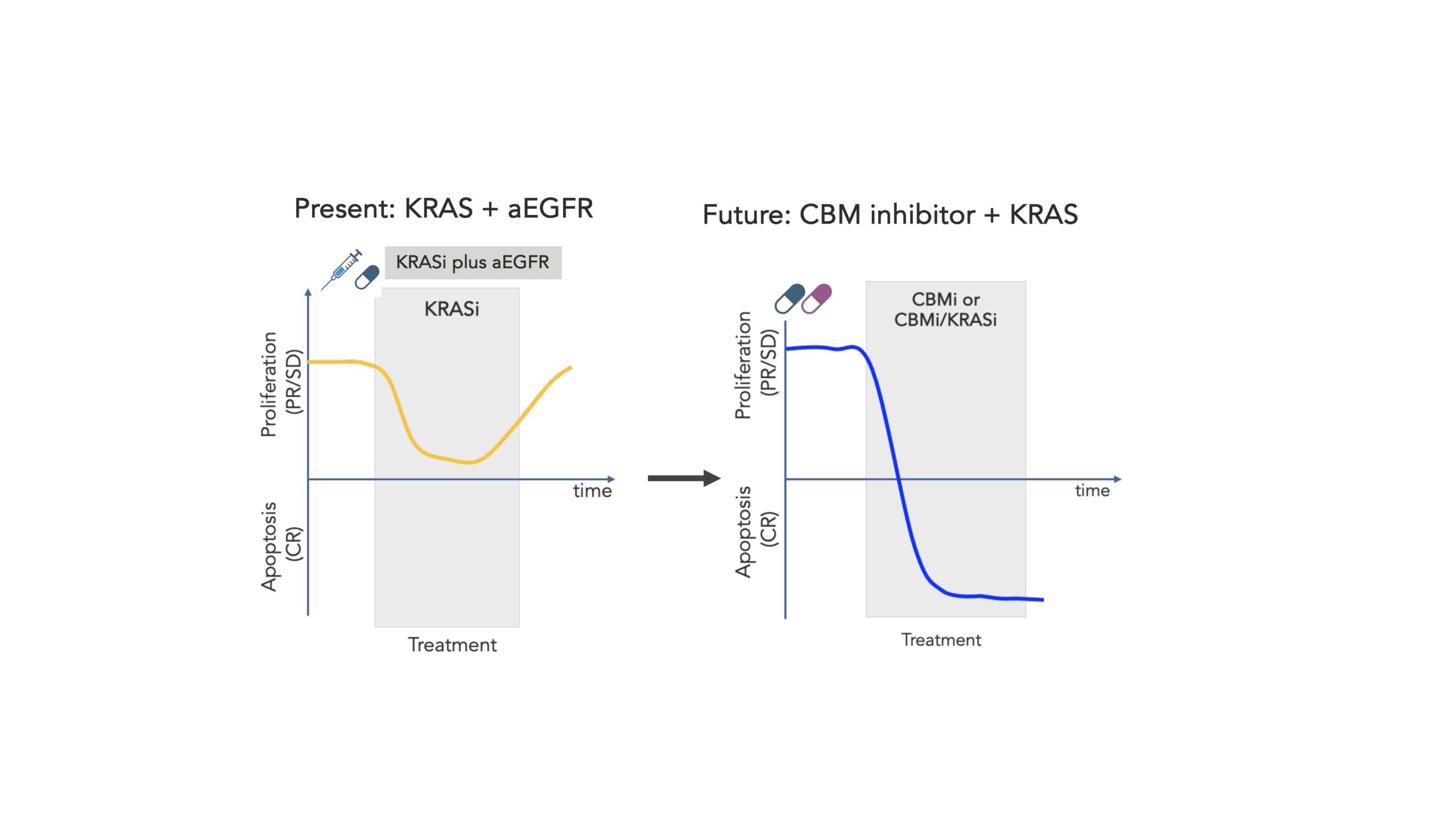
While the targeting of a complex like the CBM signalosome may be a step up in complexity, we know that even this alone may not be sufficient, as difficult-to-treat cancers like CRC tend to further outwit and evade even the shiniest and newest cancer weapons. So perhaps even more encouraging is the pre-clinical data we’ve demonstrated with our compounds when dosed in combination with KRAS inhibitors. While only in the early days of pre-clinical exploration, we’re encouraged by the promising activity of this combinatorial approach, and as we progress toward the clinic we are excited to further explore the potential synergistic effect enabled by such a combination, as well as the potential for such a combination therapy to further evade the resistance mechanisms that can rapidly emerge in these types of cancers.
As we look to the future, we can forecast the combination of a CBM inhibitor and a KRAS inhibitor as the foundation for a new treatment paradigm in KRASG12X CRC, buoyed by strong mechanistic rationale, promising pre-clinical data showing synergistic activity, and a non-overlapping safety profile. And importantly for patients, the small molecule modality would allow this combination to be orally administered, offering convenience for patients.
Taking a step back, it’s hard not to think about the profound implications revealed by our findings to date. The disease landscape, within oncology and well beyond, is littered with diseases in which the simple, straightforward, and dare I say logical solution is limited or ineffective. More often than not, complex problems require complex solutions. And perhaps, with the targeting of molecular complexes, we’re beginning to scratch the surface of one such approach – and we’re eager to continue to explore these potential implications through this program, and well beyond.