Family Oddball, and a New Class of Safe Oral Autoimmune & Inflammatory Disease Treatment with Blockbuster Potential
By Jeb Keiper, CEO of Nimbus Therapeutics, as part of the From The Trenches feature of LifeSciVC.
On day one of the JPM 2021 conference, CEO Giovanni Caforio of Bristol Myers Squibb – inheritor of Celgene’s former first presenting slot – took the virtual stage to highlight BMS’s biggest new products.
At the top of the list was deucravacitinib (née BMS-986165), an allosteric inhibitor of tyrosine kinase 2 (TYK2) that BMS projected would reach sales of $4B by 2029. This eye-popping forecast reflects the significance of BMS’ Phase 3 data (here and here) not just for psoriasis, which the company plans to submit an NDA for this year, but also potentially numerous other autoimmune diseases. With multiple BMS trials ongoing in psoriatic arthritis, lupus (SLE), Crohn’s disease, and ulcerative colitis, 2021 will be a pivotal year for seeing whether selective allosteric TYK2 inhibitors are not just better JAK inhibitors, but rather a new class of medicines for the autoimmune space as a whole. As a developer of our own selective allosteric TYK2 inhibitor at Nimbus, poised to begin Phase 2 shortly, let me share some perspectives.
The recent excitement, and BMS’ gusto, around deucravacitinib is set against the backdrop of the known safety concerns of many JAK inhibitors. Pfizer’s pan-JAK inhibitor tofacitinib (Xeljanz) was first approved in 2011 for rheumatoid arthritis and received the dreaded ‘black box warning’. Recent real-world evidence just disclosed validates the many safety concerns for patients taking tofacitinib (here). And indeed, it’s a hallmark of the ‘jakinibs’ in that they have been labeled similarly, as a class. Lilly’s baricitinib (Olumiant), Abbvie’s upadacitinib (Rinvoq), and Galapagos’ much-tortured filgotinib all get referred to as the ‘same JAK class,’ and the approved labels all share similar black box warnings.
So why then the excitement about deucravacitinib?
Simply put, the hope of an entirely new class of oral therapy that can hit the same pathway as the jakinibs but do so safely. Ultimately the claim that this will be a ‘new class’ of drugs, the ‘tykinibs,’ will be adjudicated in label discussions this year between BMS and the FDA. Still, in advance of all the TYK2 milestones ahead, I’d like to share some background on TYK2, and some of the challenges that have been overcome to develop selective inhibitors for it.
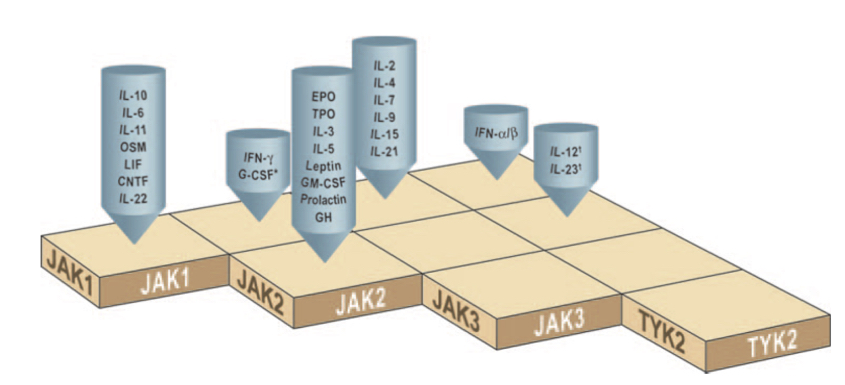
Let’s start with the fundamentals of the 4 JAKs: JAK1, JAK2, JAK3, and TYK2. (TYK2, the black sheep of the family, wasn’t named JAK4 only because it was discovered first, before the dimeric nature of JAK signaling was fully elucidated.) The JAKs form important signal transduction pathways for a range of intracellular signaling. This October 2020 Nature Reviews Drug Discovery article by Zarrin, et al., does a great job describing and illustrating the many pathways as well as pharmacological interventions currently being studied in the space – a perfect primer for those eager to dive in further.
TYK2 by and large regulates the IL12, IL23, and Type I Interferon pathways, paired with either JAK2 or JAK1. The chart below is a handy reference to JAK pairing and the various cytokine signaling pathways controlled – e.g., if you want to inhibit IL10 signaling, JAK1 is all you need; for erythropoietin receptor (EPO), you’d inhibit JAK2 alone.
While inhibiting JAKs can bring beneficial effects in autoimmune disease, there are on-target mechanistic safety issues with doing that. Take for example ruxolitinib (Jakafi), which inhibits JAK2 effectively, and causes cytopenia. Cytopenia is what you are after if treating diseases like polycythemia vera or certain types of myelofibrosis. However, hitting JAK2 in other diseases where you don’t have out-of-control proliferation can cause unwanted cytopenias.
To overcome the safety limitations of the pan-JAK inhibitors, selective second-generation JAK inhibitors (i.e., baricitinib and upadacitinib) were meant to improve the balance of safety and efficacy. Unfortunately, the challenge in drugging the JAK family has been achieving enough selectivity to avoid safety signals. Over the last decade, small steps toward more selective JAK inhibition beyond tofacitinib have been taken, however progress has been slow. The challenge has been around the fundamental protein homology of the catalytic kinase domain on the JAKs. The video below shows the catalytic domain structures of the 4 JAKs superimposed on each other and zooms in on a catalytic binding site molecule (tofacitinib in this case). Look at the subtlety of conformational differences amongst the four JAKs – the takeaway here is that they are nearly identical!
Source: Nimbus Proprietary Video
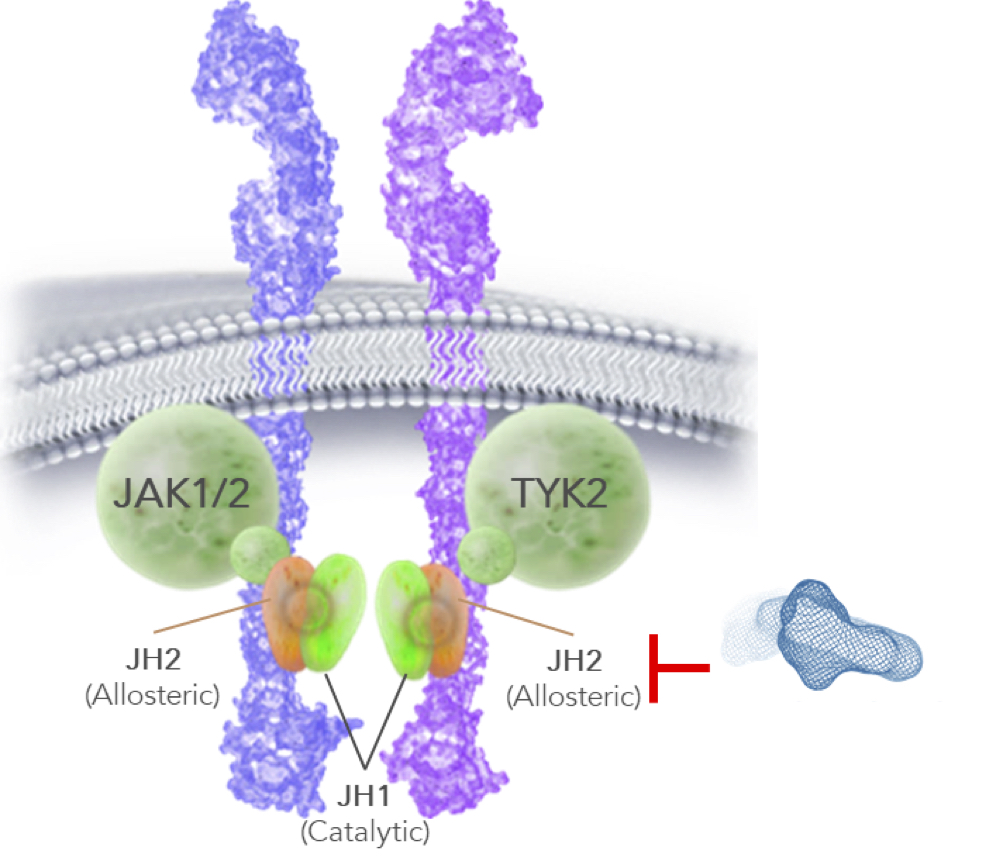
The breakthrough with TYK2 came in targeting the allosteric site, also known as “pseudokinase,” “JH2,” or “regulatory domain,” which has allowed BMS (and Nimbus, to an even further extent) to push selectivity beyond anything achievable in targeting the catalytic site. This allosteric site has long been known to exist, however binding a drug to it that results in signal inhibition was only discovered in the last few years and gave rise to the allosteric TYK2 programs at BMS and Nimbus. The result of these allosteric programs has been to drive up selectivity for TYK2 by orders of magnitude over all other previous approaches.
So what does that selectivity look like in practice? Below is a set of figures illustrating the human dosing range of a representative set of small molecule JAK inhibitors, showing both maximal concentration (Cmax) and average blood concentration (Cav). What is clear from this illustration is that all of the agents shown below are in some ways multi-JAK: All of them hit the IC50 (50% inhibitory concentration) of more than one JAK family member within their clinically relevant dosing window (and none of them effectively inhibit TYK2). This creates the proverbial double-edged sword of these first-generation jakinibs: The drug will capture some anti-inflammatory activity of those JAKs, but also pick up some of the liabilities. For example, the JAK1 activity of upadacitinib drives its primary therapeutic benefit, however hitting JAK1 also inhibits oncostatin-M (OSM) signaling which in turn has been hypothesized to cause the creatinine protein kinase elevations observed in the clinic (here). Less frequent but more hazardous risks like thromboembolic events have plagued these pan-JAKinibs, without yet having full causality mapped (here).
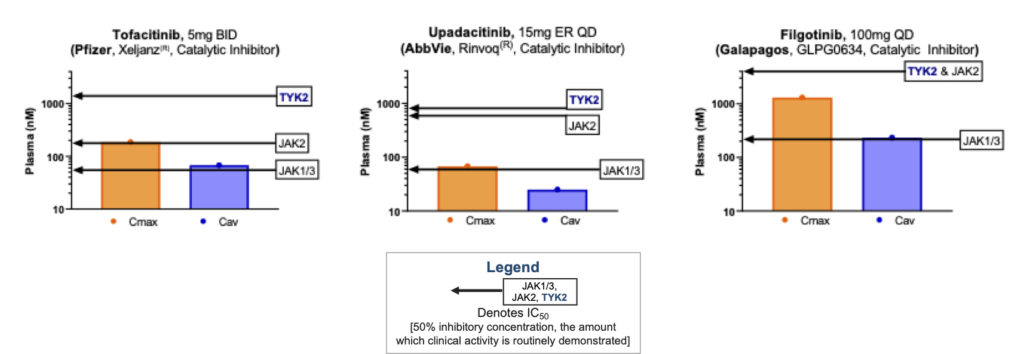
Source: Internal Nimbus analysis of publicly available information. For approved agents, approved dose regimen used. Maximum concentration (Cmax) and average concentration (Cav) in human plasma and cellular potency.
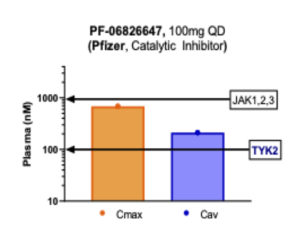
Many emerging JAK inhibitors call themselves TYK2 inhibitors to perhaps be associated with the TYK2 ‘class’ which does not have the baggage of a history riddled with safety concerns, but in fact such programs may not be truly selective against TYK2. Pfizer calls its PF-06826647 compound a ‘TYK2 inhibitor,’ but as we look at the data, it is not selective, as it clearly hits other JAKs along with TYK2 (see figure below). The “JAK1,2,3” line in this case indicates the level at which profound clinical activity against those targets would occur, so the Cmax just below that amount still creates significant pan-JAK inhibition. Furthermore, in this figure 100mg QD is illustrated, though the Pfizer team also have tested 400mg QD in the clinic – raising the concentrations fourfold higher. In their recent manuscript describing PF-06826647’s clinical results in psoriasis (here), Pfizer authors state, “the transient decrease in reticulocytes suggests that PF-06826647 might inhibit erythropoietin-JAK2 signaling at higher doses. This observation is consistent with the inhibitory potency of PF-06826647 in an enzyme assay, in which TYK2 IC50 is 15 nmol/l and 74 nmol/l against JAK2.” They go on to say that perhaps inhibiting JAK2 won’t be that bad as they could gain more inhibition of IL12 and IL23 by hitting both TYK2 and JAK2. Of course, that is possible, but that compound will carry forward the risks of non-selectivity through development.
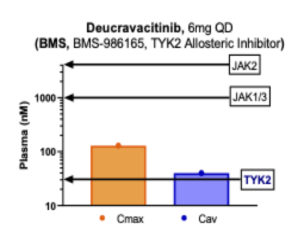
Clean selectivity on only one of the family members escaped drug developers’ hands until deucravacitinib. At 6mg QD, deucravacitinib very clearly only hits TYK2 with the rest of the JAK family members spared by high margins (see figure below). Deucravacitinib is the first truly selective TYK2 inhibitor due to the fact that it targets the allosteric site. Due to improvements in the Nimbus chemical series, our TYK2 allosteric inhibitor extends these margins on JAK1, 2, and 3 even further.
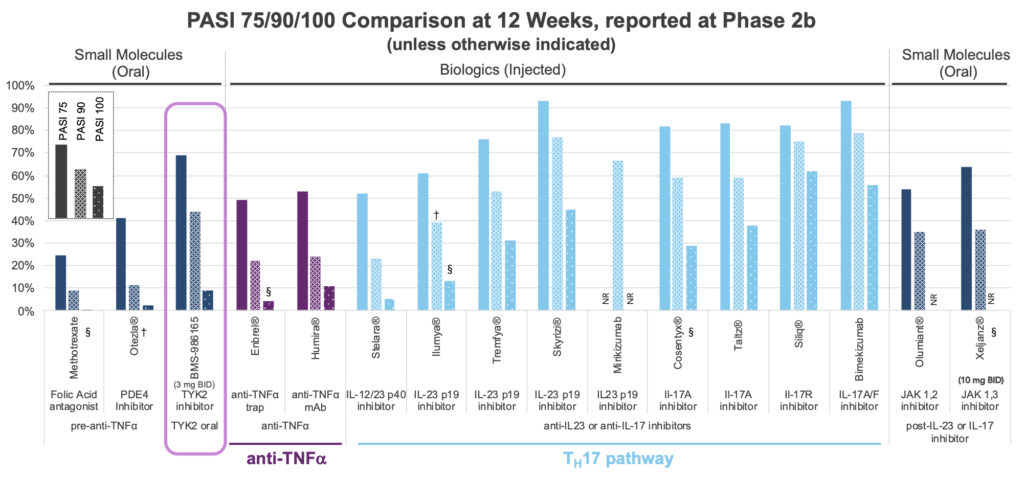
Until the BMS paper on deucravacitinib’s Phase 2b results in psoriasis (NEJM, Sept 2018), the notion of getting profound clinical activity by just hitting TYK2 and not hitting any other JAK family member was simply hypothetical. Those Phase 2b results were striking – a simple oral small molecule was producing monoclonal antibody-like efficacy to rival some of the IL23 / IL17 mAbs. The figure below expands on that comparison. The bars represent the proportion of treated patients achieving a change in psoriasis area and severity index (PASI) of 75, 90, and 100% (equating to complete clearance of the skin) across a range of therapies. To the left of highlighted deucravacitinib is apremilast (Otezla), which despite modest efficacy generates more than $1B per year in sales. Otezla was sold to Amgen in 2019 for $13B, due to competition with deucravacinitib. By combining the safety and simplicity of Otezla with potential mAb-like efficacy, selective allosteric TYK2 inhibitors have given analysts enough fodder to begin projecting real trouble for Otezla.
What drives further excitement is TYK2’s potential beyond psoriasis, into other autoimmune and inflammatory diseases (unlike the PDE4 apremilast). Deucravacitinib is being studied in the inflammatory bowel diseases (Crohn’s and ulcerative colitis), lupus (SLE), and psoriatic arthritis (PsA).
At the time of this writing, BMS has not yet disclosed the detailed results of their Phase 3 psoriasis program, but suffice it to say, all eyes will be on those data when they are shared at the American Academy of Dermatology’s virtual conference in late April 2021. It’s an incredibly exciting time to be working on selective TYK2 inhibitors, and a hopeful juncture for patients with autoimmune disease as well. With luck, this year will see the decisive evidence that marks tykinibs such as deucravacitinib and Nimbus’ own clinical candidate as a distinctive new class of therapeutics.
Viewed from that lens, maybe the namers of TYK2 were prescient not to name the protein JAK4 after all. Sometimes being a family’s oddball has its benefits.
Thanks to Alan Collis, Annie Chen, Josh McElwee, Abbas Kazimi, and Lisa Raffensperger for their insights and edits to this blog. Further acknowledgement goes to the patients and healthcare workers who have continued clinical investigation throughout this pandemic, and the vast array of colleagues, collaborators, and academics who continue to advance science that helps design breakthrough medicines.