By Arthur Tzianabos, CEO of Lifordi Immunotherapeutics, as part of the From The Trenches feature of LifeSciVC
Given everything going on in our world right now, I’ve been thinking a lot about the state of biotech innovation…particularly as we wrestle with the potential impact of cuts in NIH funding –what’s been called ‘the lifeblood of innovation’ in our industry. Truth be told, I considered making this the subject of my blog. I thought it might be interesting to share the negative impact it will have on innovation from my perspective of 15 years working in academia and even longer in industry as a biotech operator and more recently as a life science VC. But much has been written about this, and I am sure it will continue to be the subject of more articles, opinion pieces, debates, and new policies. So, I thought I’d share a different take on biotech innovation instead.
Circumventing the Wheel
How many times have we said, “Let’s not reinvent the wheel”? Let’s leverage what’s already been discovered and apply those learnings to forge a new path. This ‘circumventing the wheel’ requires navigating around the roadblocks that hampered earlier drug development efforts to bring new treatment options forward for patients.
In this vein, what if you combined a decades-old drug and a decades-old approach to deliver a simple, yet elegant solution to patients…think peanut butter and chocolate…or ‘hybrid vigor,’ as someone I recently described this concept to called it. Hybrid vigor is the phenomenon where offspring of diverse parents exhibit superior traits compared to the parents.
A Magic Bullet for Oncology
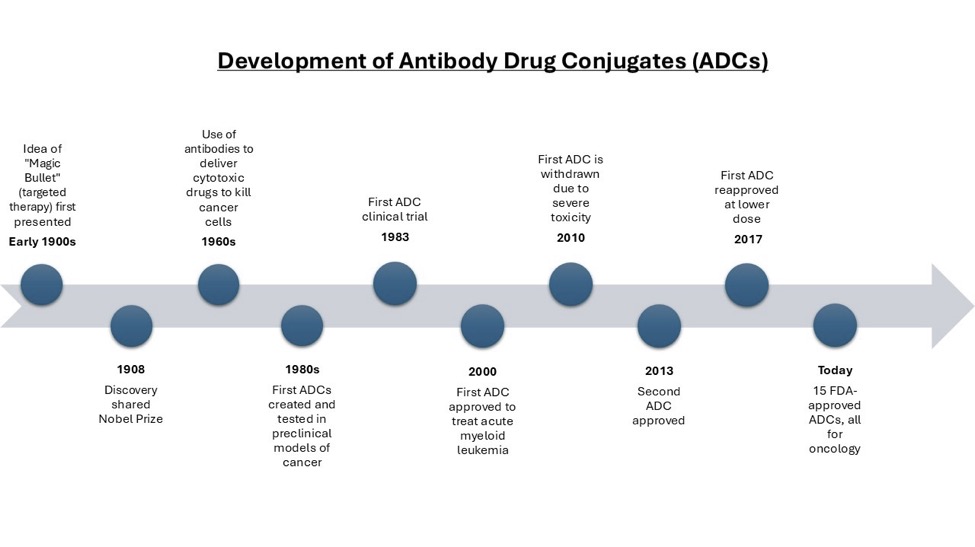
In the early 1900s, German scientist Paul Ehrlich first presented the idea that antitoxins, or antibodies, could be used to fight toxins released by bacteria. He proposed that it may be possible to kill disease causing agents without harming the host, what he referred to as a ‘magic bullet’. This approach shared the Nobel Prize in Medicine in 1908. It wasn’t until the 1960s that researchers began to use antibodies to deliver cytotoxic drugs to kill cancer, and later antibody drug conjugates (ADCs) were created as ‘magic bullets’ for delivering targeted cancer therapies. ADCs are treatments comprised of three components: a drug payload joined by a linker to an antibody.
While Ehrlich has been credited with conceiving of the concept underlying ADCs, the first ADCs were not successfully created and tested in preclinical models until the 1980s. Today there are 15 FDA-approved ADCs for oncology.
Companies developing ADCs for cancer treatment have begun to shift their focus to addressing the unmet needs in autoimmune and inflammatory diseases (I&I). Venture capitalists and Big Pharma are also increasing investment in companies developing ADCs. Evercore ISI reported that from 2023-2025, both the number of private financing deals and the highest deal value were attributed to oncology first, followed by I&I, and then other therapeutic areas. During this period, over $10 billion was invested in I&I, nearly $2 billion of which was in ADCs. Additionally, the percentage of deals involving ADCs has doubled over the past year.
ADCs are here to stay. Numerous white papers, scientific publications, presentations, and industry news articles focus on ADCs. Spanning therapeutic categories and medical disciplines, ADCs have been broadly featured in gynecologic, thoracic and pulmonology medical journals, Chemical Engineering and Science and industry trades like BioSpace, just to name just a few. The more we learn about ADCs, including how to find the right target for a particular disease, and how to produce them more efficiently, reliably and cost-effectively, the more we can unlock their full potential.
A ‘Magic Bullet’ for Autoimmune and Inflammatory Diseases
In 1948, doctors at the Mayo Clinic administered the first dose of steroids to four rheumatoid arthritis patients. The results were spectacular. Here’s how the Journal of Royal Society of Medicine described it:
“Extraordinary events occurred… The pain relief and functional benefits were of a different dimension. One totally bedridden patient was able to get out of bed and attempt to dance.”
While the patients relapsed completely when the supplies ran out, the team knew they were onto an important breakthrough. Two years later, they won the Nobel Prize in Medicine. The first steroids (also called corticosteroids or glucocorticoids) were approved by the FDA a few years later, hydrocortisone in 1952 and prednisone in 1955.
Today, steroids are widely used to manage many acute and chronic inflammatory disorders, including asthma, rheumatoid arthritis, vasculitis, eczema, allergic reactions or anaphylaxis, inflammatory bowel diseases, leukemias and lymphomas.
But…here’s the problem. Efficacy comes at a high physiological cost…
These drugs are extremely toxic. When steroids are administered, particularly at high doses or for extended periods, they remain in the body long enough to impact multiple organs and systems beyond their target.
Steroids are one of the most common reasons for admission to hospitals for drug related adverse events. In fact, a recent article in a medical journal asked: Would glucocorticoids be approved for clinical use if discovered today?
And despite this, today….
- 50 million people or 1% of the world’s adult population is taking glucocorticoids long term
- The global market for glucocorticoids is approximately $5 billion and growing
- Steroid toxicity is linked to 40% of the cost of healthcare for patients on glucocorticoids
Steroids are both a blessing and a curse.
What if you combined these two Nobel Prize-winning discoveries of ADCs and steroids to deliver the effectiveness of steroids without the toxicity? You could radically change how immune and inflammatory diseases are treated by allowing this powerful class of drugs to be used to its full effect.
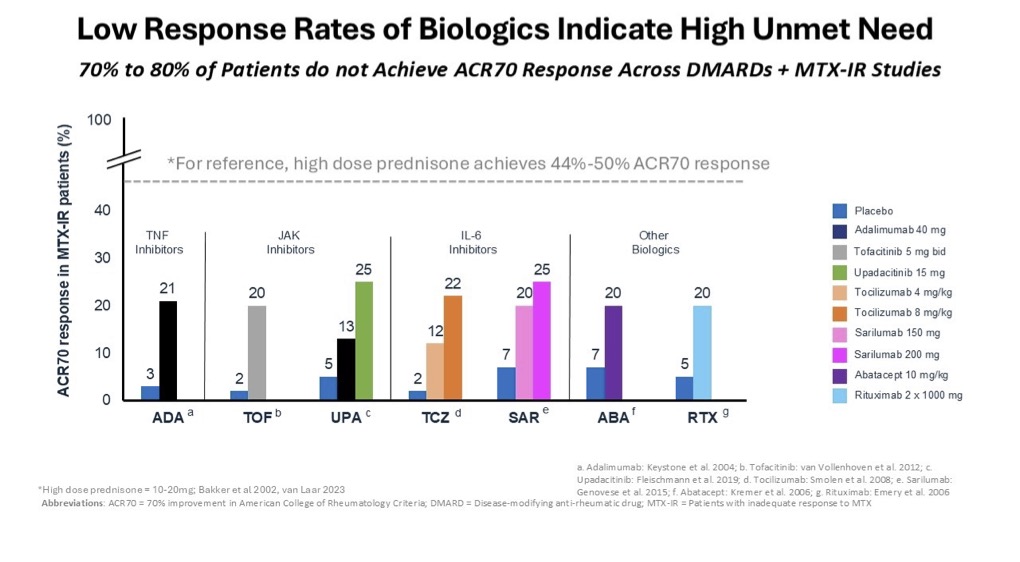
But wait….haven’t new modalities such as biologics, which include the anti-TNFs, JAKs, IL-6s, etc., solved the steroid problem?
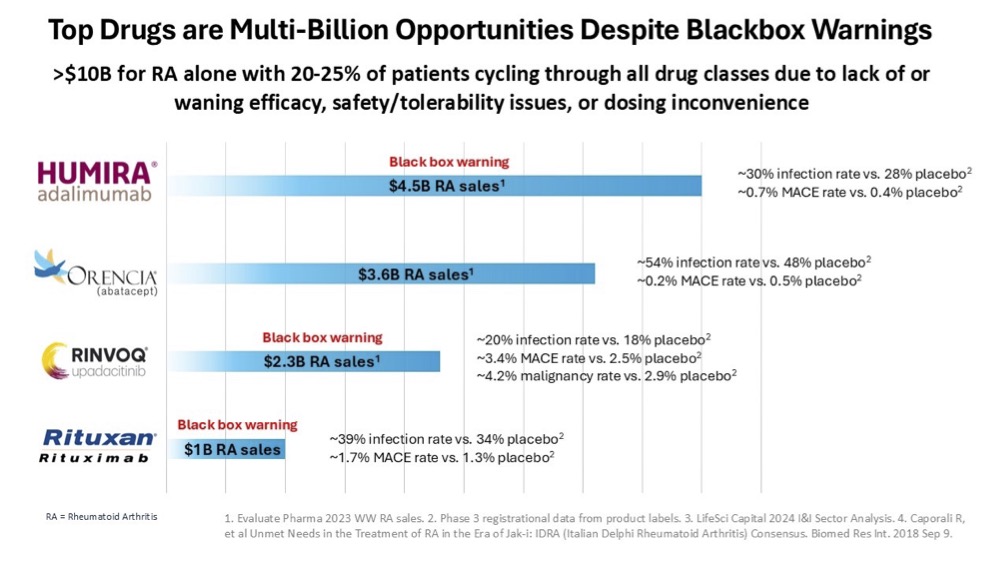
Unfortunately, the response rates of biologics are not as effective as steroids and some of them have black box warning labels. Despite not achieving the desired response rate and the serious safety concerns, biologics are still among the top selling drugs across a variety of indications. This serves to highlight the large unmet need that exists in treating autoimmune and inflammatory diseases. Take for example, a prevalent autoimmune disease like rheumatoid arthritis, which accounted for more than $10 billion in sales in 2023.
Realizing the Vision
Using an ADC to deliver a potent steroid directly to immune cells would enable you to harness the effectiveness of steroids and avoid the off-target toxicity that has hampered their broader or longer-term use. While this ‘magic bullet’ is not an entirely new concept, the success of this approach lies in identifying the right target and establishing the right formulation that delivers an effective, safe and convenient treatment for autoimmune and inflammatory diseases.
The Right Target
VISTA (V-domain Ig suppressor of T cell activation) is a cell surface protein selectively and highly expressed on immune cells, including myeloid, T cells and plasma B cells. Its unique properties, including its rapid internalization and accumulation of the drug payload, make it an ideal ADC target. A VISTA-directed ADC carrying a potent steroid demonstrated quick uptake by immune cells and a short serum half-life, measuring just hours vs. the days that is characteristic of serum residency for many ADCs. In other words, this ADC’s serum residency inside immunes cells far exceeds its exposure in serum. As a result, there is much less concern that the ADC would be floating around in circulation for too long, potentially breaking off, releasing drug elsewhere in the body, and causing systemic toxicity.
Since VISTA is doing all the work to rapidly internalize the ADC, there’s no need for the monoclonal antibody to have any pharmacologic function –-leave that up to the effectiveness of the steroid.
The Right Formulation
All FDA-approved ADCs are administered through intravenous injections, and all are approved for cancer indications. This is no surprise given how most cancer drugs have been developed and used in clinical practice. However, developing ADCs for autoimmune and inflammatory diseases practically necessitates an alternative approach that supports subcutaneous (SC) injection and perhaps oral delivery in the future. Formulating a complex, three-component ADC (never mind one with a drug to antibody ratio (DAR) of 8), was thought to be impossible. It took years to accomplish this and then to demonstrate that it can be manufactured reliably and with the required stability and scalability for clean, efficient SC steroid delivery. This VISTA-directed, DAR8 ADC carrying a steroid showed superior efficacy with no toxicity in multiple preclinical models of disease and inflammation, and with lower doses and less frequent administration. We at Lifordi Immunotherapeutics are currently completing IND-enabling studies with this innovative approach to solving the steroid problem using a novel ADC that is poised to enter the clinic and generate initial data this year.
And that’s just the beginning….
Early research shows that this VISTA-directed ADC approach also works with different payloads, including ASOs, siRNA and other nucleic acids. From peanut butter and chocolate to any ‘hybrid vigor’…innovation can come in a variety of flavors.
Reminds me of a great U2 song: The Sweetest Thing