This blog was written by Jason Gardner, CEO of Magenta Therapeutics and former EIR at Atlas Venture, as part of the From The Trenches feature of LifeSciVC.
We are excited to open the first page of Chapter 2 at Magenta today. Since our public launch in November 2016, we have been working hard to reach our vision of changing patients’ lives, particularly in autoimmune and genetic diseases, by transforming transplant medicine through innovative new stem cell science. It’s been a whirlwind of generating data, collaborating with expert scientists and physicians, and testing our ideas with new investors and potential partners.
We unveil some of this progress today with a trio of major milestones in Magenta’s journey. This next chapter will propel Magenta’s platform and programs with support of a robust Series B financing, transform the company into a clinical stage organization through an in-licensed asset from Novartis, and bring us closer to patients with a ground-breaking partnership with the premier patient-focused organization in transplant medicine, Be The Match BioTherapies.
But first, let’s recap our story so far.
It’s been a fast six months from when we closed our Series A round, which in turn was preceded by a 12 month prologue of careful diligence, team recruitment and seed capital allocation with Atlas Venture and Third Rock Ventures working in tandem here. Our mission was resolute: to change that conversation between the patient and physician around the decision to undergo a transplant; shifting the focus from the risk of the procedure to one much more about the potential curative clinical benefit. We also built a grand plan to extend that conversation and the benefits of transplant to many other disease settings, such as multiple sclerosis, scleroderma, Crohn’s disease, sickle cell anemia, metabolic diseases and more.
The clinical data from a set of transplant trials during the past 6 months have shown that the benefits in autoimmune diseases are profound, and in some patients, quite spectacular. Comparing the durable effects of a single transplant in multiple sclerosis patients by the stringent no-evidence-of-disease-activity (NEDA) composite measurement, shows 78-83% of transplanted patients achieved NEDA at 2 years in comparison to 13-46% of patients receiving currently approved medicines in a post-hoc analysis (manuscript here). We only have data from small numbers of transplanted MS patients, who typically had highly-active relapsing remitting disease, although recent meta-analyses from 15 studies with 746 MS patients supports similar conclusions and highlights the 2.1% mortality risk of the procedure (manuscript here). Larger, randomized trials comparing transplant to current standards of care are underway, and results should be very revealing.
Systemic scleroderma (SSc) is an aggressive autoimmune disease where many more patients have been transplanted in randomized Phase II and III trials. SSc is difficult to treat with a very challenging prognosis similar to some cancers (5 year overall survival rates of 50%). Transplanted patients showed significant improvement over standard of care in disease remission and overall survival with long-term follow up (review here), and more recently the European clinical rheumatology organization, EULAR, has recommended this treatment for rapidly progressive SSc, even though toxicities remain (here). With the US-based SSc trial recently delivering similar results (abstract here) as the earlier EU trials, there appears to be a paradigm shift occurring in this area of autoimmune disease medicine where rebooting the immune system via transplant provides transformational benefit for the most seriously affected patients.
Yet, all that glitters is not gold. This remarkable clinical progress is muted by side effects and toxicity that limit the application of this transformative medicine. Magenta aims ultimately to solve these challenges with new science and the first end-to-end patient-focused strategy. We are developing a portfolio of products that can be deployed in different disease settings from autoimmune diseases to genetic diseases to blood cancer and across all aspects of the patient’s transplant journey which together providing a new tool-box of modern medicines for physicians
As the clinical world of transplant in autoimmune diseases was moving quickly, we were advancing at a swift cadence at Magenta.
Enthusiasm was high as Chapter 1 began: coming out of our seed “stealth” phase, we were already moving quickly with 20 people at the company and our scientists generating early data in our labs. Stem cell assays are hard, requiring significant expertise and time, so the first objective was to establish well-validated systems built on reliable biology. Strong science is at the heart of Magenta, and these results would prove to be very catalytic as they demonstrated that our expertise and platform could deliver into our three major program areas: patient preparation through targeted antibody conditioning, stem cell harvesting with biologics triggering physiological mechanisms, and stem cell expansion using key targets with robust effects.
The interest in Magenta’s science and vision was evident through meetings over the past year in the parallel worlds of medicine and business. We met with physicians at the top transplant conferences, including the American Society of Hematology, and the European Bone Marrow Transplant meeting. These physicians were positive about the science and proposed potential collaborations and clinical paths forward. We also talked to investors and companies at the JP Morgan Healthcare conference who expressed interest in the vision which led to many follow up discussions.
We were executing on an ambitious Series A plan. The data were rolling in and we were attracting excellent scientists to the company. Yet during the external conversations with this diverse set of groups, we were getting asked lots of questions but many focused on a simple proposition:
What can we do to help you go faster?
Recognizing that the opportunity to innovate in transplant medicine is vast, and coupled with broad external interest crystalizing rapidly around our vision, these factors enabled us to accelerate the development of Magenta and bring additional resources, expertise, a clinical program and a partner into a bold plan. There are three sets of new characters that are critical to our story in Chapter 2:
The new investors:
The Series B round goes beyond providing capital to build out Magenta and drive our programs to the next key milestones. It enables the company to work with well-networked investors who believe in our vision and want to transform medicine over time. We were keen to balance the team of investors and bring onboard groups that were going to help Magenta tackle some of the next stages. We work as one team at Magenta from the board room to the lab, and our investor roster now includes early-stage company builders, Third Rock Ventures and Atlas Venture, a long-term strategic, GV (previously Google Ventures), funds with close-ties to our academic roots, including Partners Innovation Fund and Access Industries (who support the Blavatnik Accelerator program), and now cross-over investors such as Casdin Capital and another blue chip public investor. This syndicate was complemented by Be The Match BioTherapies (more below), with their first investment.
The new asset:
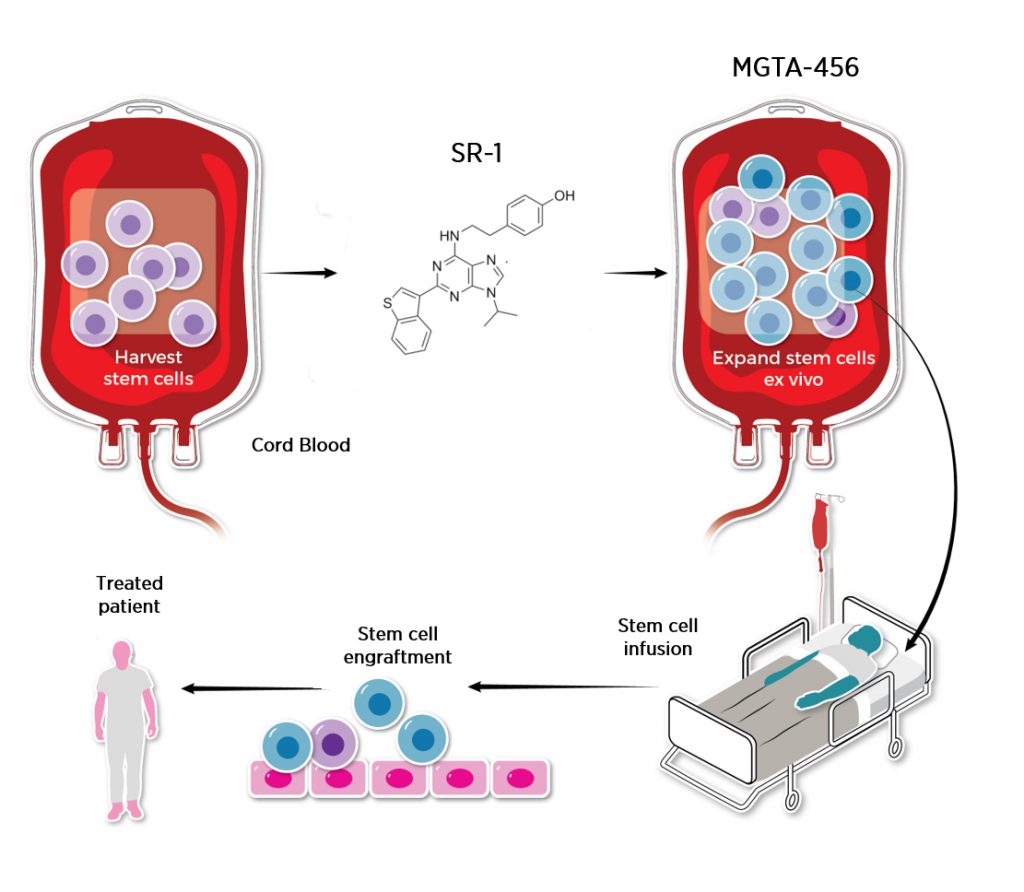
We built Magenta on an R&D platform and have some very exciting data on our own assets. Another feature of the Magenta strategy is to be a magnet for innovative new science in the transplant field, and we had reviewed a lot of the cutting-edge science in the field as we created the company. We are making strong headway on our antibody-targeted conditioning assets and new mobilization approaches. One of our three core program areas is stem cell expansion. The thesis here is that by increasing engraftable cell numbers for transplant that not only will disease effects be improved but finding a matched donor with a sufficient stem cell dose for a patient will be easier. This has been a “holy grail” for the field. It is particularly important for banked cord blood as a source of stem cells, as the cell numbers can be too low for effective transplants.
We have been generating some interesting results with Magenta compounds that can increase the numbers of stem cells prior to transplant, and were comparing them to programs that had been developed by other groups. One of the most-studied pathways for expansion is the Aryl Hydrocarbon Receptor (AHR), which controls stem cell self-renewal, and the most advanced compound targeting AHR, called SR1, was being used at Magenta as a positive control for our preclinical assays. The key stem cell science on AHR had been discovered by Novartis in an extensive phenotypic screening campaign and a set of compounds was published in Science in 2010 (manuscript here). The leading scientists on that team included Mike Cooke and Tony Boitano, both of whom we had recruited to Magenta last Summer, Mike as our CSO and Tony as Head of Stem Cell Biology. The lead compound had progressed into clinical trials with expanded cord blood transplant in blood cancer patients, and this development program was led by Bastiano Sanna, who we had also recruited to Magenta in early 2016 as COO.
The match of the science and expertise at Magenta was tantalizing.
Given the strategic fit with our vision, the quality of the fundamental science and as the emerging clinical data from the first 17 patients the Phase I/II trials looked promising (manuscript here), we started a conversation with Novartis to transition the program to Magenta. This program would leverage the existing expertise within Magenta, would align nicely with the product portfolio goals we set out from the beginning, and would allow Magenta as a company help patients across a number of diseases much sooner. With the close ties between some of the Magenta team and the asset, the Business Development team at Magenta aptly named the deal “Project Reunite.” After conducting our due diligence and negotiating the terms and contract with Novartis, we are well underway with recruiting a clinical team and the operational plan for new trials is maturing as we transform into a clinical stage organization. There will be new data on this program, now termed MGTA-456, presented at an upcoming conference, and we will be developing it in a focused way to hopefully provide transformative clinical benefit to patients in new disease settings beyond blood cancers. This development strategy is ideal for a biotech company with our focus on transplant medicine, and we continue to pursue novel approaches in this broad area of expansion. We also continue to actively explore additional innovative science across all program areas including conditioning and mobilization.
The new partner:
Stem cell transplant medicine is an extensive, global enterprise with over 60,000 patients undergoing the procedure in over 500 centers each year. With a big vision to grow the future state of transplant for more patients in broader diseases, it was clear that Magenta would need a credible partner early on to collaborate. Be The Match BioTherapies (BTMB) (website) are the interface between the National Marrow Donor Program (NMDP) and Be The Match, where they facilitate the collection, tracking and delivery of cell therapies around the world. BTMB and Magenta represent natural partners with a shared mission to improve transplant medicine, and we had engaged in discussions on how we could work together even before we launched the company last year. The long-term partnership gives Magenta the opportunity to leverage BTMB’s cell delivery capabilities and relationships, including patient outcomes data derived from the NMDP. Through these capabilities, Magenta will be able to study the effects of transplant through mining of the patient database, while also looking forward and working with BTMB to design the clinical studies for our medicines as we seek to shape the future of patient care.
Looking back on the discussions with investors, Novartis and BTMB, it was clear that having a biotech at the nexus of this field to drive new innovation would be exciting. From a Magenta perspective, delivering on the promise of our vision in broad therapeutic areas is going to take strong partnerships and focused execution. One aspect that was satisfying was that these groups were also convinced that the science we are pursuing is feasible. In other words, we are fully leveraging existing biology and clinical knowledge and marrying that with modern advancements in technology and biological understanding. It’s an ambitious goal, but also scientifically and clinically very reasonable and tractable.
We are delighted with the progress in these early days, and this momentum will be vital as we work with our new protagonists in Chapter 2 to build Magenta. We still have many more chapters ahead in our story, and it will be fascinating to see how we might shape the future of medicine for patients with this innovative approach.