This blog was written by Rosana Kapeller, CSO of Nimbus Therapeutics, as part of the “From the Trenches” feature of LifeSciVC.
It is the holiday season and as we gorge on delicious and heavy food, and inevitably resolve to eat less and exercise more after the new year, it seems an appropriate time to reflect on the progress that is being made in the development of therapeutic agents to treat non-alcoholic steatohepatitis (NASH). NASH, a condition linked to the “diabesity” epidemic, affects millions of people worldwide and is expected to surpass HCV as the leading cause of liver transplants in 10 years. Despite the high unmet need, there are currently no approved drugs for treating NASH and the current recommendation is lifestyle modification, which is only partially effective.
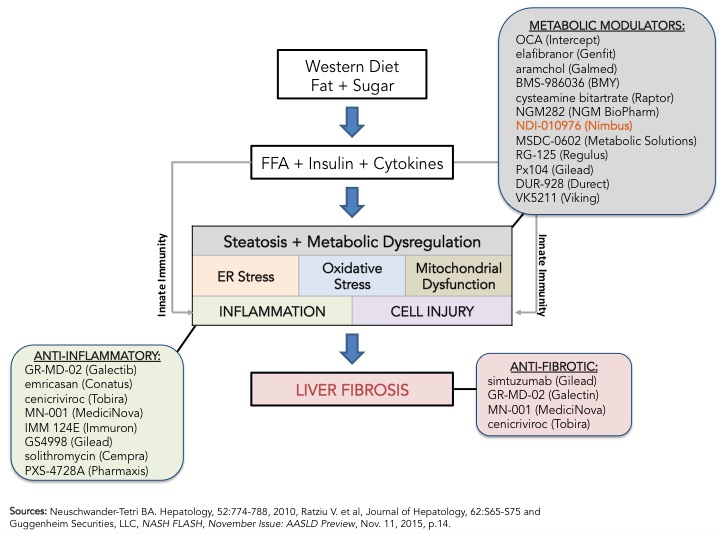
The path to registration is still unclear but that is not stopping drugmakers from investing heavy in NASH as seen by the number of studies registered in clinicaltrials.gov for NASH or NAFLD; 119 total, 45 of which are currently open and recruiting. My prior blog on NASH, From Crawl to Sprint; the Race to Treat NASH, and references within, provides an overview on why NASH is hot and is getting so much attention from industry and regulators. I wrote that blog, just after EASL’s 50th International Liver Congress held in Vienna last April and the biggest announcement was Intercept’s OCA pivotal Phase 3 clinical trial design (REGENERATE) announcement. Fast forward 6 months, to AASLD’s Liver Meeting, Genfit announced its pivotal Phase 3 clinical trial design for elafibranor, a PPAR ?/? agonist, in NASH. Intercept and Genfit, the front-runners in the race to treat NASH, are closely followed by Gilead’s simtuzimab and GS4998, Tobira’s cenicriviroc, Conatus’s emricasan and others. As Bill Tanner and his team at Guggenheim Securities point out in the November 2015 “NASH Flash” update (see figure below), the mechanisms of action vary greatly including metabolic modulators, anti-inflammatories and anti-fibrotics, and the current thinking is that combination therapy may be required to impact the fundamental pathogenic processes that lead to NASH, namely increase in free fatty acids leading to inflammation and fibrosis. What is clear now, after the initial sprint, is that this is not a high speed, short distance race, but a marathon and the front-runners are setting the pace.
What have we learned in the past 6 months?
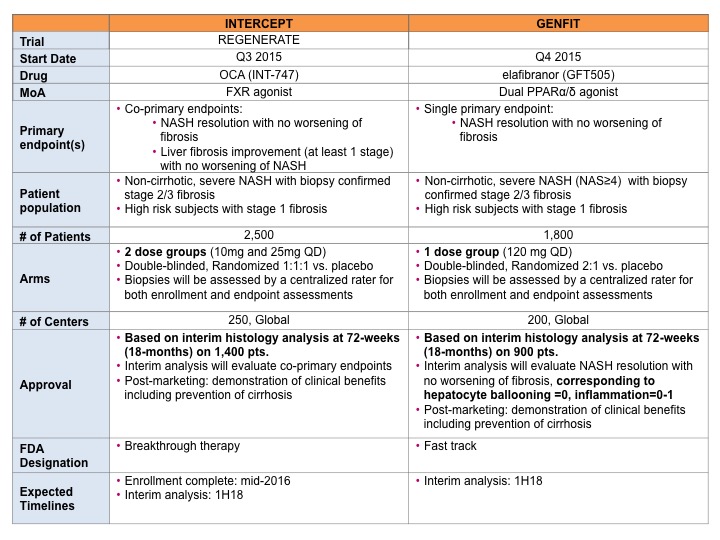
Regulatory path for approval is becoming clearer given Genfit’s Phase 3 clinical trial design: Although the long awaited guidance from the FDA has yet to materialize, it is becoming clear that the regulators may accept the resolution of steatohepatitis with no worsening of fibrosis as the primary endpoint for initial market approval, as discussed by Lara Dimick-Santos from the FDA, but will require post-marketing assessment of clinical benefits, for example evaluating progression to cirrhosis and other liver-related events, for full approval. She also recommended concomitant submission of Phases 3 and 4 designs to the FDA (note: her remarks reflect her own personal opinion and should not be construed as official guidance from the FDA). One big change of note is that “resolution of steatohepatitis” will only be considered valid if lobular inflammation and hepatocyte ballooning decrease, not just steatosis, as measured by the NAFLD activity score (NAS), a widely recognized histologic measure of disease activity for NASH. NAS components include steatosis (0-3), lobular inflammation (0-3) and hepatocyte ballooning (0-2) scored during the histological analysis of a liver biopsy. Total scores of 3-4 are considered borderline for NASH, whereas scores of 5-8 are diagnostic of NASH. Until 2 weeks ago, the understanding was, that a decrease of ? 2 points in the total NAS score, regardless of what component was being scored, indicated improvement of NASH.
Interestingly, Genfit’s pivotal Phase 3 clinical trial design of elafibranor in NASH (here) has incorporated all the above guidelines and surprisingly did not follow Intercept’s lead on co-primary endpoints (NASH resolution with no worsening of fibrosis and liver fibrosis improvement with no worsening of NASH). Instead Genfit chose a single primary endpoint, “NASH resolution with no worsening of fibrosis, corresponding to ballooning=0, inflammation=0-1.” In my opinion, this is one of the biggest differences and may benefit Genfit. In addition, Genfit’s trial, with only one dose, will require a reduced number of patients (1800 total with interim analysis based on 900 patients vs Intercept’s 2500 total with interim analysis based on 1400 patients) and therefore may allow Genfit to catch up with Intercept (interim analysis for both companies expected in 1H18). This is a clear win for Genfit.
Summary of Pivotal Phase 3 Clinical Trial Designs:
Tough terrain ahead: The race, however is hitting some snags and it is not clear what the finish line will look like. For example, the recent interim results from Intercept’s Japanese partner, Sumitomo Dainippon Pharma, on the 72-week Phase 2 placebo-controlled, dose ranging (10mg, 20 mg, 40 mg) trial of OCA in 200 Japanese patients with NASH (>5 NAS score) was rather disappointing. Only patients treated with the 40 mg dose of OCA achieved statistical significance on the primary endpoint as compared to placebo (a reduction in NAS ? 2 pts with no worsening of fibrosis) and no difference was seen in fibrosis improvement in any OCA groups compared to placebo. These results contrast with the Phase 2 FLINT results in western subjects, which showed a statistically significant difference for NASH subjects taking 25 mg OCA vs. pbo on both endpoints (45% of subjects on 25mg OCA compared to 21% on placebo and 35% of subjects on 25 mg OCA vs. 19% on placebo for the same primary endpoint and fibrosis improvement, respectively). The reason behind these unexpected results is not clear and may be due to differences in population baseline characteristics and the size of the study, which was significantly smaller than the Phase 2 FLINT study. Final results from this study is anticipated in 2H16. The lesson here is that NASH is a complex and heterogeneous disease, and clinical trial designs need to consider this inherent variability, as well as potential differences between populations to adequately power them and the findings in one population may not be translatable into a different one, and that will need to be accounted for in the clinical trial designs to power them appropriately.
Advancement of Non-invasive Diagnostics: One of the most pressing needs in the field is the development of reliable, non-invasive diagnostic tools. NASH is an under-diagnosed condition since it requires a liver biopsy to confirm the diagnosis, and once it is confirmed, patients are reluctant to undergo serial biopsies for management, which right now is life-style modification. The other issue with biopsies is that they are extremely variable. Think about it, the liver is the second largest organ in the body, measuring approximately 21-22 cm across, 15-17 cm vertical height, and 10-12 cm front to back. A typical liver biopsy is 2 cm in length and about 1.6 mm in diameter. It is like the proverbial “finding the needle in the haystack”, since the disease does not affect the whole liver homogeneously, and can lead to the wrong diagnosis and staging of the disease (variability is up to 40%).
What is needed are non-invasive diagnostic tools that reflect the overall state of the liver. Right now they are divided into two modalities: (i) imaging and (ii) blood- based markers.
- Image-based: Imaging techniques can vary from a simple widely available fibroscan all the way to complex MRI-based techniques that are just available at a single center. Imaging is expensive, and studies are still ongoing to fully validate the different methodologies required to measure either liver fat or fibrosis. No imaging tools exist today that reliably detect inflammation or all three processes together. In addition, there are no longitudinal studies comparing the results of biopsies with imaging studies and currently the regulators are not inclined to replace the “gold standard” liver biopsy with imaging as a surrogate endpoint for registration. There is also controversy on what to measure in imaging studies. For example: is the reduction of liver steatosis a good marker of response? Rohit Loomba from UCSD, would argue that is the case. At AASLD’s liver meeting, Patel and Loomba presented a poster (# 913: Non-invasive quantitative decline in liver fat content on MRI and histologic response in nonalcoholic steatohepatitis: A secondary analysis of MOZART trial) showing that decrease in liver fat content as measured by MRI-based proton density fat fraction (MRI-PDFF) correlated with histologic response in NASH. Fifty patients with biopsy-proven NASH were randomized and treated for 24 weeks with ezetimibe (10 mg, oral/day) or placebo, 35 of these patients underwent paired biopsy and MRI-PDFF. The histologic responders (10 patients) showed statistically significant reduction in liver fat content by MRI-PDFF that translated to a 29% decrease whereas the non-responders (25 patients) showed a 2% increase in liver steatosis. If this finding holds true, this is a major step forward in showing that (i) MRI-PDFF is a reliable methodology for the follow up and management of patients with NASH, and (ii) resolves a big controversy in the field on whether or not liver fat content reduction correlates with improvement in NASH. In addition to MRI-PDFF that measures fat content, magnetic resonance elastography (MRE), which measures the stiffness of the tissue, is being used in the clinic as a noninvasive alternative to liver biopsy for staging hepatic fibrosis.
The hope is that in a few years imaging will be a staple in the diagnosis and management of NASH as it has occurred for other conditions that also required invasive diagnostic procedures.
- Blood-based markers: The rapid progress in imaging technologies does not replace the need for rapid, reliable and inexpensive blood-based diagnostic tools in addition to routine liver function tests, fibrosis and inflammation markers. Many companies, together with academic collaborators, are investing significant time and resources to develop serologic methodologies that have both high specificity and sensitivity. To date, the search has been disappointing. Markers such as fibrosis-4 (FIB-4), platelet ration index (APRI), AST, ELF and others have been used concomitantly with non-alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) and liver biopsy/NAS in several trials including Intercept’s FLINT trial. At AASLD’s liver meeting, it was reported by Drs. Arun Sanyal and Stephen Harrison, that although FIB-4 and APRI showed good specificity, they lacked sensitivity. A median 10% improvement in FIB-4 or a 34% improvement in APRI values at 24 weeks were “associated with at least one-stage improvement in fibrosis at 72 weeks, as seen on biopsy” and the changes were statistically significant (here, here). However, if fibrosis increased there was little change in either marker. Other serologic markers such as LOXL-2 levels, seem to correlate well with advance stages of fibrosis as reported by Dr. Stephen Harrison (poster # 1435: Serum lysyl oxidase-2 levels correlate with fibrosis stage in patients with nonalcoholic steatohepatitis) in Phase 2b trials with simtuzumab. Genfit announced last September (here) the identification of “two specific miRNAs species within the top 3 most powerful diagnostic markers of NASH” and that proprietary algorithms combining the miRNAS with known markers of NASH have been developed and will be deployed during the Phase 3 studies. Genfit has established collaborations with other groups studying NASH and is open to partnerships to further develop its proprietary diagnostic tool.
The need is great for the development of these non-invasive tools and the success of advancing therapies in NASH may hinge on that, however it is a very challenging proposition due to the heterogeneity of NASH, one of the reasons why the diagnosis of NASH still relies on histological changes since there is a lack of knowledge on what constitute the different forms of NASH (diet-induced, associated with diabetes, genetic, viral, etc), and the natural progression of the disease. Therefore progress on both fronts is required for the development of reliable non-invasive diagnostic tools.
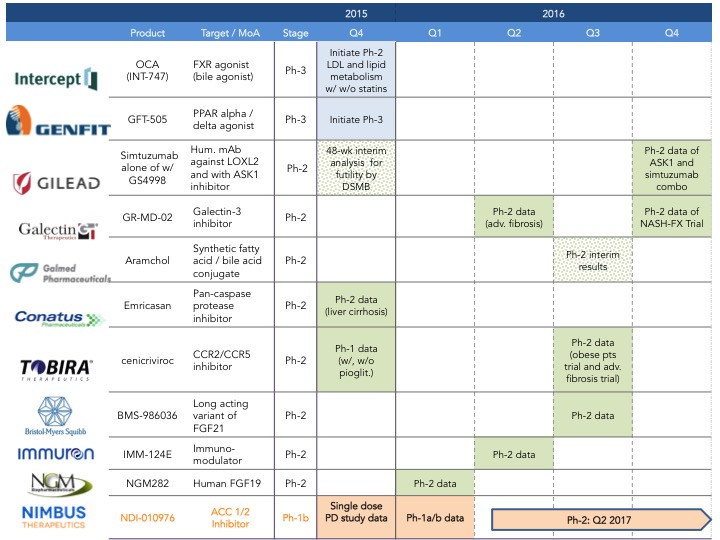
What to expect in 2016: There is no doubt 2016 will be a busy year for NASH, since several companies are expected to announce their phase 2 data and others are initiating new trials with novel compounds (2016 development catalysts below). For example, Intercept just announced last week (here) that is initiating Phase 1 clinical trials for their dual FXR/TGR5 agonist (INT-767).
Nimbus plans to be at EASL in Barcelona to present our Phase 1 data on NDI-010976, a potent and selective allosteric acetyl-CoA carboxylase inhibitor that in pre-clinical studies has been shown to decrease steatosis, inflammation and fibrosis in mechanistic models of hepatic steatosis and liver fibrosis (here).
I wish everyone a merry and happy holiday season. Careful what you eat, your liver will thank you!