This guest blog was written by Troy Lister, Head of Chemistry, and Cristina Larkin, Chief Commercial Officer, of Spero Therapeutics, as part of the From The Trenches feature of LifeSciVC.
A national security threat equivalent to terrorism? This is how Britain’s chief medical officer described drug-resistant bacteria. Dr. Thomas Frieden, recently retired director of the CDC, referred to drug resistant bacteria as one of the most serious health threats that “has the potential to harm or kill anyone in the country, undermine modern medicine, to devastate our economy and to make our health care system less stable.”
The CDC estimates that every year, at least 2 million people are infected with drug resistant bacteria and that the number of deaths resulting from these infections is equivalent to one fully loaded jumbo jet crashing each week. We have also seen a lack of novelty in treating these infections. In fact, 40 years have lapsed since a novel antibiotic class was approved for use against Gram-negative infections.
The lack of novelty is by no means an indication of lack of effort devoted to this cause. Indeed, across the public, private and academic industries many extensive and protracted drug discovery endeavors have been undertaken, only to come up short of a novel therapeutic. The reason for these numerous failures lay not in a lack of quality molecular targets or even a lack of inhibitors of such targets, but rather the inability to get these good inhibitors to their targets in Gram-negative bacteria. In fact, it is predominantly the physiology of Gram-negative bacterium, specifically the make-up of the outer-membrane of these bacteria that has hindered innovation. Whilst Gram-positive bacteria possess a single phospholipid cell membrane, Gram-negative bacteria have both a phospholipid inner membrane (akin to the Gram-positive membrane) and an outer membrane bilayer composed primarily of phospholipid at the inner surface and lipopolysaccharide (LPS) at the outer leaflet. It is this layer of highly polar, negatively charged LPS that excludes many excellent target-based inhibitors including to a trove of wonderful, clinically useful Gram-positive antibiotics from entering Gram-negative bacteria to do their work. Unfortunately, a distinct incongruence between chemical properties required for Gram-negative penetration (both membranes) and that imposed by many of the known (and novel) bacterial targets means that simple (or complex) medicinal chemistry tactics have not been able to provide a solution.
Spero Therapeutics hopes its adjunctive, Potentiator approach (SPR741) to LPS disruption will be the solution.
SPR741-First new approach to gram-negatives in 40 years
SPR741, Spero’s lead Potentiator candidate, is currently progressing through Phase 1 clinical trials. SPR741 is an analog of the well-known natural product antibiotic, Polymyxin B (PMB). PMB, as a single agent, is an efficient killer of Gram-negative bacteria, but unfortunately, this molecule is also associated with severe, dose-limiting kidney toxicity, and the chemical attributes that make PMB an efficient antibiotic are complicit in the observed toxicity. SPR741 was specifically designed to utilize one of PMB’s favorable attributes, namely interaction with Gram-negative LPS, while being devoid of the safety liabilities. SPR741 does not directly kill bacteria, but very potently interacts with LPS, essentially disrupting this barrier to entry for many antibiotic classes. Indeed, preclinical studies of SPR741 in combination with many Gram-positive antibiotics have shown success in reducing the bacterial burden of infections caused by several common drug-resistant pathogens,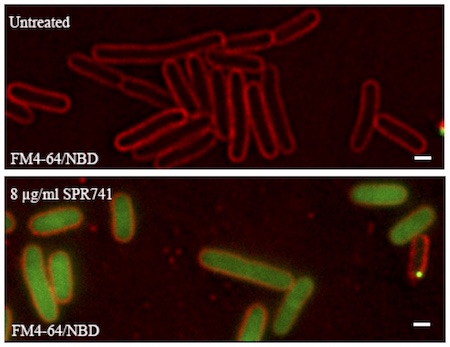 The mechanism of action (MOA) of SPR741 is captured in a stunningly visual representation below. In this experiment, E. coli bacteria have had their outer membrane labeled with a fluorescent red dye (you can see the characteristic rod shape) and these bacteria are ‘swimming’ in a pool of green fluorescently tagged azithromycin. On the top, SPR741 is not present, and azithromycin is unable to penetrate the bacterium and concentrate enough to be visualized. On the bottom, a small amount of SPR741 has been added, and now the azithromycin enters the cell, concentrated and is visualized. The inability of azithromycin to enter Gram-negative bacteria renders it incapable of killing these organisms. Simply, the addition SPR741 allows azithromycin to freely translocate the outer membrane and ultimately kill Gram-negative bacteria.
The mechanism of action (MOA) of SPR741 is captured in a stunningly visual representation below. In this experiment, E. coli bacteria have had their outer membrane labeled with a fluorescent red dye (you can see the characteristic rod shape) and these bacteria are ‘swimming’ in a pool of green fluorescently tagged azithromycin. On the top, SPR741 is not present, and azithromycin is unable to penetrate the bacterium and concentrate enough to be visualized. On the bottom, a small amount of SPR741 has been added, and now the azithromycin enters the cell, concentrated and is visualized. The inability of azithromycin to enter Gram-negative bacteria renders it incapable of killing these organisms. Simply, the addition SPR741 allows azithromycin to freely translocate the outer membrane and ultimately kill Gram-negative bacteria.
Spero has also investigated the impact SPR741 has on the cell surface structure of Gram-negative bacteria using atomic force microscopy (AFM). AFM employs an exquisitely sensitive technique to scan the surface topology of a biological specimen. Spero employed this technology to map the impact exhibited by SPR741 on the topology at the outer membrane of Gram-negative bacteria. The image below on top shows the natural, relatively smooth topology of the cell envelope of an E. coli bacterium. Below, the E. coli was exposed to a small amount of SPR741 leading to significant perturbation of the surface as seen by the dramatic change in topology. This disruption allows an opening for the adjunctive antibiotic to enter the cell and exert its antimicrobial activity.
Spero has taken a very broad approach to understanding and characterizing the capacity of SPR741 to potentiate other agents. Combinations of SPR741 with a host of generic and novel (clinical and pre-clinical) molecules have been assessed for enhanced antibiotic activity. These activities have demonstrated a vast capacity for SPR741 to potentiate activity, which we classify into two categories. First, molecules that are dramatically potentiated from a complete lack of Gram-negative activity to a profile encompassing activity against multiple Gram-negative bacteria, including drug-resistant variants at clinically relevant concentrations. Examples include large and/or lipophilic molecules like the rifamycins, pleuromutilins, macrolides, mupirocin and fusidic acid whose activity can be increased up to 5 orders of magnitude. Second, are a group of molecules that penetrate the Gram-negative outer membrane reasonably well, but that can still see benefit from the addition of SPR741 by expanding the coverage of organisms that may be intermediate or resistant to the antibiotic alone. Examples include the well-established and trusted cephalosporins, carbapenems, and quinolones.
Upon successful completion of its Phase 1 evaluation of SPR741, Spero will introduce first combination product to emerge from this franchise, namely a combination of SPR741 with a generic Gram-positive antibiotic. This combination exhibits potent efficacy against life threating Gram-negative organism including E. coli, K. pneumoniae and A. baumannii, including drug resistant variants like extended-spectrum beta-lactamases (ESBLs), and Carbapenem-resistant Enterobacteriaceae (CRE). Additionally, this approach will enable Spero to leverage a deep safety database and broad usage/availability across the world in the partner antibiotic and the combination will qualify for various regulatory incentives including accelerated 505b2 filing, and Qualified Infectious Disease Product (QIDP) designation, which allows for fast track approval and 5 additional years of exclusivity.
Given the broad potentiation exhibited by SPR741, we expect this to be the first of multiple combinations to enter clinical use akin to the experience of combining beta-lactamase inhibitors with multiple generic and novel beta-lactams.
Certainly significant focus and energy has been spent over the past 2 years taking SR741 from concept to clinical stage asset, but Spero has also spent time building knowledge and deployable technologies to further refine our understanding of structure-activity and structure-toxicity relationships of these unique molecules. In so doing, Spero has assembled a platform of assets capable of addressing multiple areas of unmet need.
The future of SPR741, and Potentiators broadly, is an exciting one and all of us at Spero are excited at the potential to significantly impact the practice of infectious disease medicine and provide clinicians and patients alike more options.






