This blog was written by Samantha Truex, Atlas EIR and CEO of Quench Bio, as part of the From The Trenches feature of LifeSciVC.
Recently I read the article What’s Wrong With Me? that I saw re-posted on Twitter. It’s a 2013 New Yorker article chronicling one woman’s journey through years of multi-symptom autoimmune/autoinflammatory disease with undiagnosed and untreated maladies. It’s a long read, but one that I could not leave unfinished as I thought about my multiple friends who followed similar paths for years, each trying to understand what caused her symptoms. Amongst my close friends are one with multiple sclerosis, one with ulcerative colitis, one with lupus and two with rheumatoid arthritis (RA). Their diseases are given these titles that imply crisp definitions, but these are heterogenous diseases with overlapping symptoms, so they are difficult to diagnose and treat. I’ll come back to what we might do to change that.
NIH classifies 24 diseases as autoimmune and estimates >23 million Americans suffer from these diseases. The American Autoimmune Related Diseases Association tracks >80 diseases with an autoimmune basis, and estimates 50 million Americans suffer from those diseases. This is a huge number of people, many of them suffering for years from insidious disorders that leave them exhausted, depressed and baffled, right along with their doctors, about whether there is anything they can do to change their condition. Lupus has been particularly challenging with GSK’s Benlysta (belimumab) as the only new drug approved in ~50 years for lupus and the only targeted drug. Unfortunately, I believe its overall efficacy is viewed as “ho-hum,” to use a technical term.
I find a glimmer of hope in the research of recent years. Since the early 2000s, anti-TNF therapies have revolutionized treatment of RA and other indications, though many patients still progress. Several drugs that target inflammatory signaling have been approved in more recent years (e.g. JAK inhibitors, antibodies to IL-1b, IL-6, IL-17 and IL-23). These may inhibit innate immunity pathways or impact the intersection of innate and adaptive immunity. They have all achieved significant efficacy in at least one indication. Novartis’ canakinumab, targeting IL-1b, has been in clinical trials for >30 indications and appears to be very effective in certain rare autoinflammatory diseases and one form of juvenile arthritis, but has yet to show a sufficient balance of efficacy with safety concerns to achieve approval in other settings. I hope there are still more indications to come with this therapy.
Several therapies in discovery and early development today directly target innate immunity. Perhaps these will work where other approaches have failed to provide relief or may work in concert with some that provide incomplete efficacy. BMS has a preclinical program targeting PAD enzymes to block inflammatory citrullination, which leads to both: 1) neutrophil extracellular traps (NETs; see Figure 1) that play a role in multiple autoimmune diseases including lupus and 2) anti-citrullinated protein antibodies (ACPA) that play a role in RA. IRAK4 inhibitors are now in the clinic, targeting a key node downstream of toll-like receptors. Multiple companies are working on cGAS/STING antagonists to block intracellular signaling that leads to excessive interferon production.
At least five companies are working on inhibitors of the NOD-like receptor NLRP3, a key target in the canonical inflammasome pathway that leads to inflammatory cell death. Pfizer previously pursued NLRP3 inhibitor MCC950/CRID3 into the clinic, but found liver tox signals in Phase 1 . CRID3 now serves as tool compound of NLRP3 inhibition that shows impact in several animal models and serves as a structural guide to the companies looking to duplicate its biological impact.
Having succeeded in preclinical stages with NLRP3 inhibitors, Jecure was acquired in November by Genentech and IFM Tre was acquired by Novartis earlier in April. At least as important as these acquisitions is the fact that IFM Tre has reached the clinic with the first NLRP3 inhibitor since CRID3, announcing the dosing of the first patient in a healthy volunteer study in late March. This is a fantastic step forward for patients suffering from many diseases rooted in inflammation, including potentially NASH, atherosclerosis and neuroinflammatory diseases such as Parkinson’s and Alzheimer’s.
In an April BioCentury article about these inflammasome approaches, Novartis declined to reveal details about its clinical plans for the IFM Tre programs, but did describe the initial path as exploratory and intended for multiple indications, like it was for canakinumab. I applaud IFM for simultaneously pursuing multiple inhibitors programs to offer peripherally-restricted, brain-penetrant and gut-targeted chemical series to Novartis. Novartis is now equipped to invest in the broad clinical investigation it has shown itself willing to pursue.
As research around inflammatory cell death has matured since the discovery of NLPR3, additional inflammasomes and other targets have been uncovered that drive chronic inflammation leading to autoinflammatory and autoimmune diseases. These include other NOD-like receptors and other cell death drivers like MLKL, RIP kinases and gasdermins – all worthy targets potentially differentiated from NLRP3 and from each other. The may have the potential to address inflammatory diseases driven by NETs and multiple types of inflammatory cell death (e.g. NETosis, pyroptosis, necroptosis). 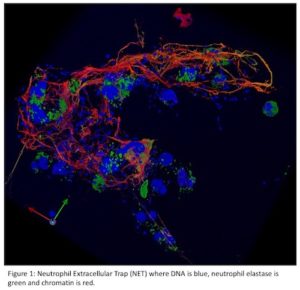
My hope for the ongoing development of all of these approaches is that we as an industry will find better ways to clinically explore the complex, overlapping and idiopathic autoimmune conditions. Most trials in the autoimmune/autoinflammatory space today are conducted by disease. Perhaps we need another approach? Perhaps there is disease overlap such that one target – and hopefully one therapy – could have a profound impact in subsets of patients from multiple diseases. Just as mismatch repair deficiency is a biomarker crossing the boundaries of multiple cancers, perhaps some subset of lupus patients and some subset of Crohn’s disease patients share a key target – and perhaps it’s one of the innate immunity targets described above. How can companies explore this without embarking on a costly and time-consuming trials to explore each of these diseases in parallel, especially when clinical research in heterogenous diseases like lupus has led to so many clinical failures of late?
According to a recent Timmerman Report, MIT’s Project ALPHA, directed by Professor Andrew Lo, looks at sophisticated assessment of success in drug development. Lo highlights that innovations in clinical trial design like “basket trials” allow for assessment of a therapeutic across multiple, related diseases. Chris Buckley, Professor of Translational Rheumatology at the Universities of Birmingham and Oxford, is exploring the possibility of just that – basket trials. He leads a program that aims to deliver “stratified pathology” in a range of immune-mediated inflammatory diseases in order to choose the right disease indication for the right drug. He is comparing history and molecular profiling data with clinical outcomes across disease cohorts.
How can basket trials be practically be implemented? We need good biomarkers that cross diseases. Dr. Buckley’s research may highlight those. Tregs are already used as a key biomarker for efficacy of IL-2 therapies. Interventional basket trials of IL-2 therapies are here and here, the latter enrolling patients across 14 diseases, measuring impact on Treg cells as the primary endpoint with assessment of inflammation markers, relapse rate and quality of life. Perhaps key biomarkers are starting to emerge in innate immune pathways, as well. For example, a 2018 Nature Reviews article on NET-driven diseases points out that low-density granulocytes (LDGs) are present in a subset of lupus, the subset of vasculitis patients who don’t respond to rituximab and a subset of RA patients, with a theory that these are the RA patients who are TNF-unresponsive. Perhaps the presence of LDGs could serve as the biomarker for a basket-trial across lupus and RA patients. The same article points out missense mutations in the genes encoding DNAse1 and DNAse3 as correlated with familial lupus. These mutations leave DNAses unable to properly clear the extracellular DNA in NETs, leading to the anti-nuclear antibodies that are a hallmark lupus. Perhaps a trial enrolling patients with these missense mutations could capture a subset of lupus patients and maybe a set of patients who suffer from idiopathic inflammatory diseases, like the author of the 2013 New Yorker article. Maybe an inhibitor of NETs will work in all of these patients from both of these theoretical basket trials.
That’s a lot of maybe’s that we need to test. I have high hopes for the future of patients who suffer from chronic inflammatory diseases with no answers, including many too many women of my age bracket. I hope that we’ll start crossing boundaries of complex diseases to illuminate the similarities across them and translate therapeutics more effectively to the appropriate patients. Those patients are waiting on us.





