By Aoife Brennan, CEO of Synlogic, as part of the From The Trenches feature of LifeSciVC
 I love to travel. While still in training in internal medicine, I earned extra money to fund trips abroad by covering for community-based general practitioners (GPs). This usually involved staying in the GP’s house for the weekend, holding the cell phone and carrying a medical bag in what I call ‘the boot’. I am a country girl at heart and driving around the Irish countryside between seeing a couple of patients at the practice was a nice escape from the city.
I love to travel. While still in training in internal medicine, I earned extra money to fund trips abroad by covering for community-based general practitioners (GPs). This usually involved staying in the GP’s house for the weekend, holding the cell phone and carrying a medical bag in what I call ‘the boot’. I am a country girl at heart and driving around the Irish countryside between seeing a couple of patients at the practice was a nice escape from the city.
A core part of the work of a weekend GP was to make any requested house calls to see patients with poor mobility or nearing the end of life. Visiting a patient at home was a memorable experience- different interpersonal dynamics, more holistic compared to an office visit. I was reminded of that experience recently when my physician husband commented on his experience as he conducts more patient care via telemedicine. He remarked that his patients are more open and asking a lot more questions than they would at an office visit.
As we deal with some of the devastating consequences of the COVID pandemic and start to look past the initial ‘acute’ response, I have been spending some time thinking about the future of medicine and of clinical trials in particular. There I see some short-term obstacles but also some real opportunities to accelerate much-needed change. Could this be a long-awaited opportunity to rethink how we conduct clinical research with the patient truly at the center?
Pre COVID-19 clinical trials were happening, things were getting done, but many stakeholders were unhappy-
Sponsors see increasing costs and timelines that are getting longer. It currently takes an average of 9 months to get an academic medical center up and running to enroll.
Sites are burdened by a deluge of paperwork, amendment after amendment, as well as supporting the increasing need for audits and on-site monitoring visits. Most are lucky if they can pay their research staff through the peaks and troughs of the workload.
Patients are frustrated accessing trials and then accommodating the demands of the long on-site visits around work and family life.
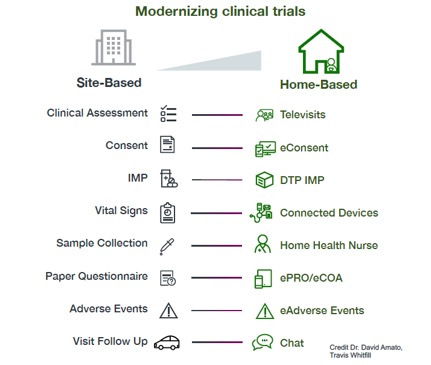
As we think about how to execute clinical trials in the context of hospitals focusing on the acute response to COVID-19 and a need for ongoing social distancing, we should think about whether home-based clinical trials could be part of the solution to some of the long term frustrations.
The resources to support home-based trials in the image below predate COVID. What has kept us from moving forward in a big way up to now is fear and inertia. We had a system we knew, we had standard risks and mitigations, the standard operating procedures were available. Who wants to spend millions on a clinical trial that ends up being un-evaluable because of a data integrity question?
Now enter COVID-19. Most medical centers have restricted or severely limited clinical trials. In a recent Biocentury report, 75% of biotechs and pharmas report significant disruption, clinical research organizations report a drop off in activity and layoffs, almost all public companies are updating their guidance around clinical data availability.
At Synlogic we are running a program to develop a treatment for PKU, a rare metabolic disease. One of the very earliest COVID emails we received was from the national patient advocacy organization (NPKUA). They wanted to know how they could help to ensure that our development program kept moving forward. Patients with other rare and not so rare diseases have also stepped forward to speak about dashed hopes of progress towards new treatments.
The net effect of disruption combined with the potential of a stuttered and prolonged return to business as usual is an opportunity for innovation. Not every trial can be run virtually but we can start to ask ‘why are we bringing the patient in for this?’. Could we see home as the default and the clinic as the exception?
As we start to implement this patient and home-centric approach, things will go wrong- investigational product will be delivered to a neighbor’s house, endpoints will ‘behave’ differently, studies will be underpowered, there will be findings on regulatory inspections. But we will learn from these mistakes and get better.
Home based studies have the potential to help trials move forward but also have the potential to address who participates in clinical research and how those individuals participate. My hope is that, similar to my experience with house calls, patients will be more engaged, ask more questions and the resulting data will be more generalizable.
Now, back to dreaming about where my next adventure travels will take me when all of this is over…..