By Tim Reilly & Jonathan Montagu, CDO and CEO of HotSpot Therapeutics, as part of the From The Trenches feature of LifeSciVC
Now 10 years into the golden era of immuno-oncology (I-O) following the first FDA approval of ipilimumab (anti-CTLA4) in 2011 and the seminal initial efficacy disclosure on nivolumab (anti-PD1) in 2012 (Topalian et al NEJM), we can reflect on how I-O has literally changed the lives of tens of thousands of cancer patients and their families.
Extension of the proverbial tail of the survival curve has provided disease relief, time, hope and, in some instances, remarkable, functional cures in an unparalleled number of tumor indications. But, as much as I-O has changed cancer care forever, things stay the same. Cancer still afflicts far too many people we know and biopharmaceutical research and development still struggles to find effective treatment options for ever evolving patients in need.
The promise of I-O was followed by the painful reminder that the next generation of I-O was not going to be a ‘gimme’ – no freebies as coaches liked to say. Despite lots of fanfare, the large number of new I-O targets and innumerable approaches to and combinations of those targets has mostly resulted in disappointment, with little to show for it other than proving the remarkable brilliance of anti-PD(L)1. Challenges abound in the lack of reliable preclinical models (reviewed in Ochoa de Olza and Garralda 2018 Annals of Oncology; Hegde and Chen 2020 Immunity), the systematic failures in clinical / translational experimental designs (retrospective summary by C. Pan et al J Hematol Onc 2020: 13, 29) and even some ill advised investments and scientific decision making.
There are too many examples to name but rather than dwell on the negatives, we need to reflect on the learnings, refocus on the positive takeaways and get back to work – cancer patients expect it. If one steps back and thinks about I-O in the context of an innovation cycle (see Figure 1 below), the remarkable success of anti-PD1 was really the result of tough learnings from predecessor approaches, even those that ‘worked’ like IL-2 and anti-CTLA4.
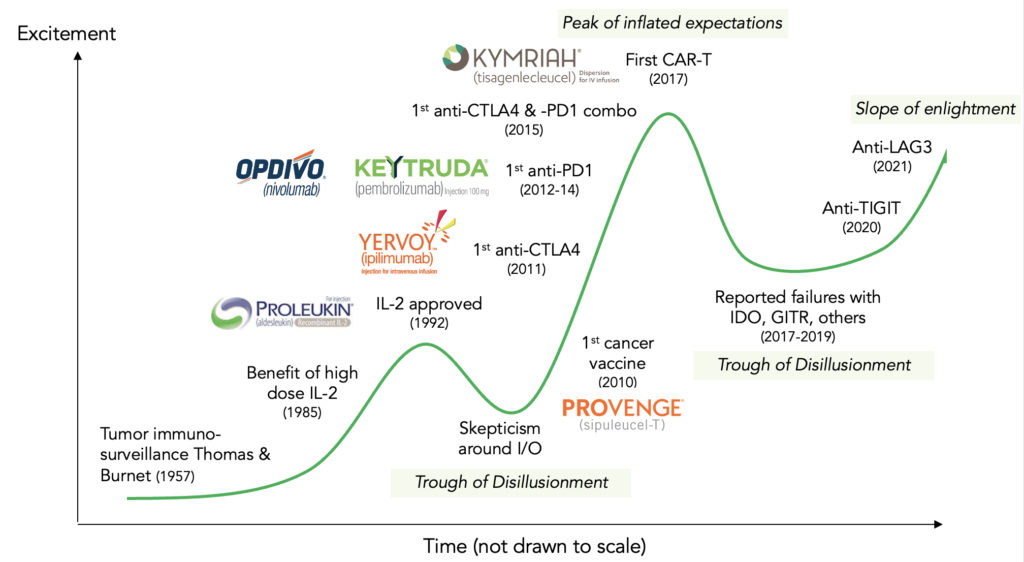 Figure 1 Innovation cycle for immuno-oncology field
Figure 1 Innovation cycle for immuno-oncology field
The so-called ‘failure’ of many subsequent mechanisms over the past several years actually provided a roadmap on how to course-correct our thinking and approaches, and recent data has given us hope once again that I-O is here to stay.
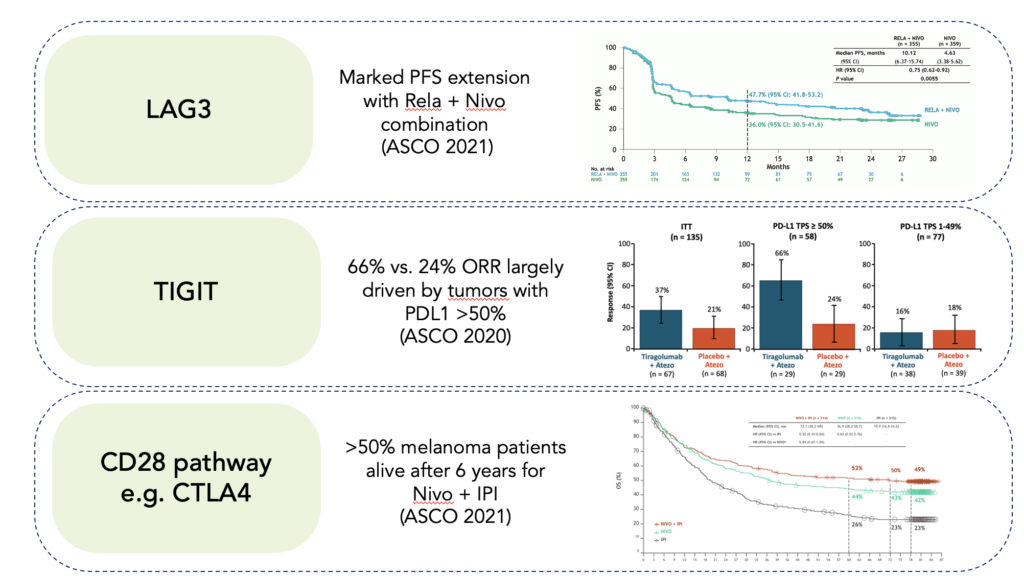
Specifically, a number of important mechanisms are showing promise (see Figure 2 below):
- LAG3: Based upon early clinical benefit of relatlimab (anti-LAG3) in a heavily scrutinized set of anti-PD1 relapsed/refractory melanoma patients ( Ascierto et al. JCO 2017), BMS committed to a large randomized controlled Ph2/3 trial. At this year’s ASCO (E Lipson et al. JCO 2021), the big reveal of that data showed the relatlimab plus nivolumab more than doubled median progression-free survival versus nivolumab alone (10.1 vs. 4.6 months) in previously untreated metastatic melanoma – a result generally on part with previously untouchable nivolumab plus ipilimumab but without the same extent of grade 3/4 adverse events.
- TIGIT: Genentech/Roche took an earlier shot on goal approach in investing in a randomized controlled Ph2 study with tiragolumab (anti-TIGIT) in combination with anti-PDL1 (atezolizumab) in non-small cell lung cancer (NSCLC) patients. With a special eye on hypothesis-driven patient population, they showed the first preliminary proof of concept that anti-TIGIT could enhance the effects of anti-PDL1 alone, with an overall response rate of 37% vs. 21% that was largely driven by effect in tumors with PDL1 >50% (ORR 66% vs. 24%).
- CD28 pathway: Driven by a spate of other successes of ipilimumab plus nivolumab, a number of novel approaches are now re-imagining how to optimally target the CD28 pathway with novel CTLA4 constructs, CD28 bispecifics and other approaches.
Figure 2 New I-O mechanisms demonstrating validation in patients
What’s different now you might ask? The tough learnings from just a few years ago reminded us to
- Have a solid hypothesis and stick with it
- Do the right experiments even when longer to do and subject to more criticism from the impatient nay-sayers; and
- Even beyond initial signals, don’t forget to continue to optimize dose, schedule, tumor types and biomarker defined patient segments.
Our take is that these recent successes are part of the natural evolution of I-O innovation. There can and should be great optimism but with a splash of realism. There is huge therapeutic promise in re-directing the immune system to detect and destroy cancer cells in even more patient segments and while many approaches may still not work, there is so much value, scientifically, clinically and financially, in trying.
Despite the enormous benefits of I-O, the majority of patients don’t respond or don’t respond long enough – and yet we know the body’s immune system is a powerful therapeutic tool. We need to be more focused than ever to target the most legitimate approaches and ask the right questions in the right ways. We need to:
- Follow the science by building upon and maximizing validated mechanisms like the LAG3, TIGIT and CD28 pathways. Importantly, testing new mechanisms and new combinations should be done in a controlled way that allows one to clearly show differentiation – equivocation is our enemy.
- Explore where the ‘ball’ is going, not where it’s been (ie, future unmet need). The greatest unmet need is for patients who relapsed on or are refractory to anti-PD(L)1 based approaches (eg, low TMB, highly immunosuppressive) and for targets that might positively impact on the multiple immune defects associated with I-O resistance.
- Actively pursue improvement of the patient experience. Amongst the many things coming from the COVID19 pandemic, the need for treatment flexibility and convenience (eg, oral agents that can self-administered at home) are paramount.
The body’s natural tumor-surveying immunity is in a proverbial fight to beat back it’s cancer cell opponents. Recent successes have reminded us that the potential is still there for I-O to bring durable benefit to more patients, and even emerging tumor cell intrinsic targets like the recent KRAS G12C inhibitor from Amgen may ultimately be best when used in concert or sequence with I-O. Our advice is don’t look away now because I-O may be the ultimate comeback kid.