By Jonathan Montagu, CEO of HotSpot Therapeutics, as part of the From The Trenches feature of LifeSciVC
The phrase ‘build it and they will come’ dates all the way back to the Old Testament, and in more recent times has been romanticized in movies like Field of Dreams, but the concept also holds true in many areas of science. Even good ideas are met with healthy skepticism for the practical and technical limitations of being able to effectively test a hypothesis.
Such is the story of targeting casitas B-lineage lymphoma-B (CBL-B) that was highlighted in a recent scientific review article published in the Journal for ImmunoTherapy of Cancer. Ryan C. Augustin, Riyue Bao and Jason J. Luke, all with the UPMC Hillman Cancer Center at the University of Pittsburgh, summarized the challenges previously encountered in targeting costimulatory pathways and the compelling scientific rationale for targeting CBL-B as a master regulator of the immune system that is downstream of proven mechanisms like CD28, CTLA4 and PD1.
Importantly, they also pointed to something that we at HotSpot Therapeutics and a small group of other researchers and companies recognized several years ago – that scientific advances have made this attractive, but once considered ‘undruggable,’ target druggable. This was recently underscored by Nurix Therapeutics who presented multiple posters at the Society for Immunotherapy of Cancer Annual Meeting on their Phase One CBL-B program. And in early January, we bolstered the potential of CBL-B inhibition by announcing clearance of our first IND based on robust preclinical data.
Learning from Past Failures to Drive Toward a More Durable Immune Response
Following the initial clinical successes of blocking inhibitory receptors, like CTLA4 and PD1, many in the immuno-oncology field explored the potential to further enhance anti-tumor immunity by simultaneously enhancing co-stimulatory pathways. The idea was simple – while one takes your proverbial foot off the brake with checkpoint inhibition, another can simultaneously boost those effects by pressing your proverbial foot on the gas with immune stimulation. Unfortunately, despite attempts at many costimulatory mechanisms by many companies, none of those approaches have proven to be clinically effective.
Augustin et al point to several potential explanations including transient expression of those target receptors and sometimes opposing effects of these pathways on various immune cell subsets. They also highlighted why targeting the CD28 pathway overcomes some of these challenges and why CBL-B modulation, downstream of CD28 and other signaling receptors with clinical validation, may be more advantageous.
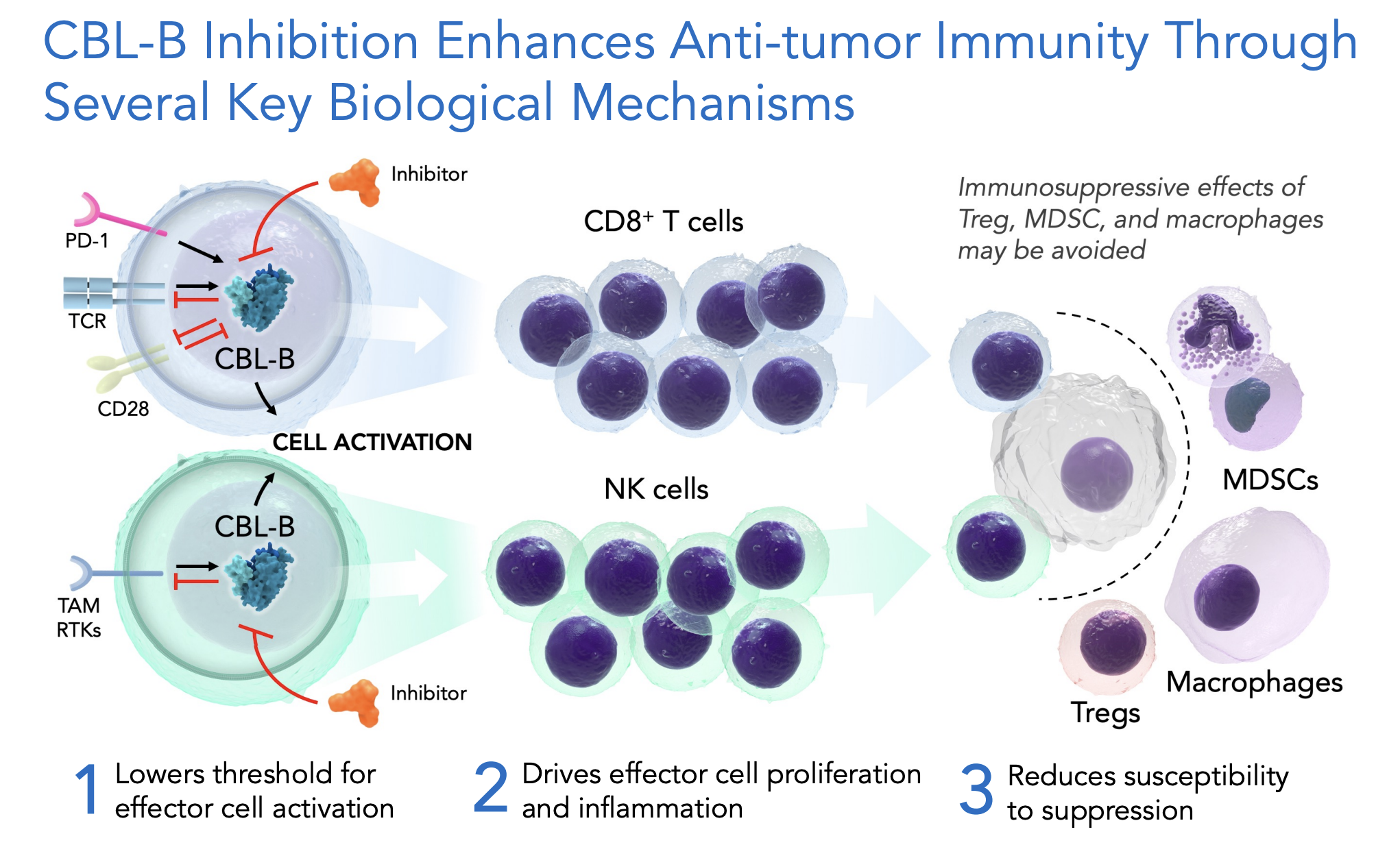
The authors highlighted how both genetic knockdown and pharmacologic inhibition of CBL-B, including with our now clinical development molecule, HST-1011, impacts a range of immune cell types to enhance immunologic responsiveness. Inhibition of CBL-B 1) makes it easier to turn on effector immune cell populations; 2) makes those cells more active; and 3) enhances their durability by making them less responsive to typical immunosuppressive mechanisms. The net result of CBL-B inhibition is that it ultimately promotes a more pro-inflammatory tumor microenvironment. Importantly, these collective attributes of CBL-B inhibition address several of the common challenges of patients’ tumors that do not respond to existing immunotherapy, or respond initially and then wane.
A New Approach to Drugging the ‘Undruggable’
In many respects, there was not a question of ‘if’ scientists should target CBL-B but more a question of ’how’ best to do so. Augustin et al summarized how novel platforms have more recently been applied to try to solve this question including CRISPR genome editing combined with adoptive cell therapy, siRNA knockdown, DNA encoded library approaches and machine learning algorithms. At HotSpot Therapeutics, we have developed a proprietary platform called Smart Allostery™ that combines computational approaches and AI-driven data mining of large and highly diverse datasets to identify functional pockets – or natural hotspots, as they are commonly called – and then target those natural hotspots with small molecules to control protein function as nature routinely does. HST-1011 is an orally bioavailable, small molecule allosteric inhibitor of CBL-B discovered via this approach, and a molecule that we hope will show the therapeutic power of targeting CBL-B for cancer patients in need of new therapies.
Thinking Smarter to Identify the Right Patients
Even with its more diverse array of biological effects, CBL-B inhibition is still not a magic bullet. Cancer is infinitely more complicated, with huge biological heterogeneity from early- to late-stage disease, between different tumor histologies and from individual patient to individual patient. Bringing the potential benefits of CBL-B inhibition to cancer patients requires us to think smarter around which patients are best suited for this approach and why.
At HotSpot, this is something we debate on a daily basis. How can we target the right patients, or at least exclude those patients unlikely to reap benefit, and thus confound early clinical data? Is there a constellation of transcriptional, protein and/or cellular markers that can be used to enrich the population towards those most likely to respond? Early insights from such an approach can be used to further refine those markers and more precisely identify the ideal patient population.
The review by Augustin et al rightly gives credence to this essential part of the debate. They even put forward a hypothesis that patients whose tumors have low to moderate inflammatory cell signatures, low to moderate antigen levels (measured by tumor mutational burden) and high CBL-B expression, might be a high unmet need population that is more likely to benefit. Only time will tell if these and other markers can enrich the population, but it is essential that biomarker-defined strategies be implemented from the earliest days of clinical study. That means partnering closely with engaged clinical investigators to recruit the right patients and to collect the right samples (including pre- and on-treatment biopsies) from the earliest days, and then integrating those complex datasets on an individual patient level to find predictors of response.
The timing could not better for this review put forward by the UPMC team. By spotlighting the challenges and the unique opportunities we have with this novel mechanism of action, Augustin et al have landed on what we believe to be the most exciting new frontier in cancer research. From our perspective at HotSpot, of course, we believe we’ve built the right type of molecule to target CBL-B therapeutically, and we are excited to see who will come along with us on this journey. As the review makes clear, there are many great minds and innovative companies exploring several aspects of CBL-B inhibition using allosteric methods. As the field continues to mature rapidly, we hope to see major breakthroughs gain momentum quickly.