This blog was written by Jason Gardner, founding CEO of Magenta Therapeutics and Atlas Entrepreneur-in-Residence, as part of the From The Trenches feature of LifeSciVC.
Imagine the conversation today between a patient and doctor on the decision to have a bone marrow stem cell transplant. The procedure could either be life-saving or life-ending, but is guaranteed to be associated with huge problems. It’s a difficult conversation that happens in hematology-oncology clinics for patients with blood cancers, sometimes for patients with genetic blood diseases but virtually never for patients battling autoimmune diseases like multiple sclerosis, scleroderma and other diseases that could cured be rebooting the new immune system. We decided to create Magenta Therapeutics to transform that conversation and make transplant medicine a real treatment choice tomorrow for many more patients.
The “medicine” in the bone marrow transplant is the hematopoietic stem cells. We each have around 11,000 of them in our marrow and they create our entire immune and blood systems, producing over 650 billion cells daily. That’s a lot of cells. They have been studied extensively with an uptick of scientific publications relating to their biology and clinical application over the last decade. Over 1 million patients have been transplanted in the past 50 years (here). They have been the shoulders that the gene therapy field was built upon as they can be genetically corrected and transplanted to treat patients with rare diseases (here).
Powerful cells, curative potential, well-studied biology, and over half a century of clinical experience. So why don’t we do more stem cell transplants?
The answer is actually very straightforward, and is crystallized in the conversations that doctors have with their patients about transplant. True, transplant can be life-saving: it may be the only hope for a patient with leukemia or another blood disorder. However, that chance at a cure comes with an incredibly high price tag. First, there are formidable risks associated with conditioning, the necessary removal of a patient’s own stem cells to make way for the new ones. Indeed, current conditioning regimens rely on decades-old science and come with a bevy of terrible side effects, including nausea, vomiting, diarrhea, bleeding, infection, and death. The life-ending risk is a sobering statistic for up to 20% of patients. Second, many patients are unable to find well-matched stem cell donors; in fact, in certain ethnic groups the odds of finding a match are only about 1 in 3. Finally, it can be challenging to collect sufficient cells for an effective transplant. Altogether, the decision takes place on a knife’s edge of benefit, flanked on both sides by tremendous risk. As a result, the discussion is never easy – and often doesn’t ever even start, especially for patients who are considered to have “chronic” or “milder” conditions like autoimmune disorders. This prohibits many patients and their families from accessing this life-changing medicine, relegating transplant to the ranks of the Hail Mary and an option of last resort in the medical armamentarium.
If we could unleash the power of stem cell medicine with a more effective transplant procedure then we could help a much larger group of patients. Beyond earlier stage blood cancers and genetic diseases such as sickle cell disease, which we know can be cured with transplant, we could improve care for conditions like myelodysplasia, where many patients are too old or frail to have a transplant. Indeed, providing a treatment option for the 100,000s of patients with few choices may become more important in our aging population. However, the biggest area of medicine that has been largely underexplored is autoimmune diseases.
Over 3,000 patients here have received a stem cell transplant over the past 20 years with stem cells to reset their immune systems and halt or reverse the disease progression. This application started out from anecdotal case reports of a subset of patients with aplastic anemia who had received a transplant for their primary disease and their doctors noticed that their secondary autoimmune disease symptoms (e.g. rheumatoid arthritis) improved (here, here). Collaborative efforts in Europe, then the US, led by pioneering rheumatologists enabled the first transplants of patients in small studies and demonstrated significant disease responses that then moved the approach larger multi-center trials (here).
The treatment paradigm is to completely “reboot” the corrupt immune system with a new one from a stem cell transplant via refreshed tolerance to self-antigens, although the exact mechanism remains incompletely understood. There is a long way to go to make this approach more feasible for patients with non-malignant diseases, but the results from the latest clinical trials indicate that like stem cell transplant has a massive impact on aggressive disease in several autoimmune settings, including a Phase III clinical trial in systemic scleroderma (here), and most recently in multiple sclerosis (here). Given the recent advances in immune-oncology and CAR-T fields, understanding the immunological mechanisms of immune reboot, tolerance and disease pathophysiology promises to be an enthralling effort for translational medicine investigation.
Magenta’s Bold Mission
So why are we launching Magenta today with a bold mission to transform this field?
We and our Founders believe that the science has reached a tipping point where there are new approaches to transplant are going to disrupt the field in the very near future. These advances are long overdue for many patients, including autoimmune diseases. With a more effective stem cell transplant and a safer procedure, we envision a future full of possibilities enabled by better ways to deliver a brand new immune or blood system.
There are three key drivers for Magenta. First, the unmet need is high for many patients who are eligible yet never receive a transplant today. Second, the science is ripe for innovating towards the clinic and expanding the eligibility with a more effective medicine. Third, we have assembled a team that has acquired real insight from stem cell drug discovery, development and clinical experience with patients that we believe will be impactful.
As we built Magenta over the past year, we also decided that the breadth of our vision would require a stem cell biology platform to run the necessary assays for discovery and development of our programs. We have assembled a unique set of methods and tools to guide our current projects in addition to delivering the next wave of programs.
The science underpinning Magenta is drawn from the academic labs of our Founders, David Scadden, M.D., and Derrick Rossi, Ph.D., at Harvard, based on work from Rahul Palchaudhuri, Ph.D., Jonathan Hoggatt, Ph.D., and Agnieszka Czechowicz, M.D., Ph.D. We have complemented this work with additional unpublished science and intend to disclose our plans as we build out our platform and programs.
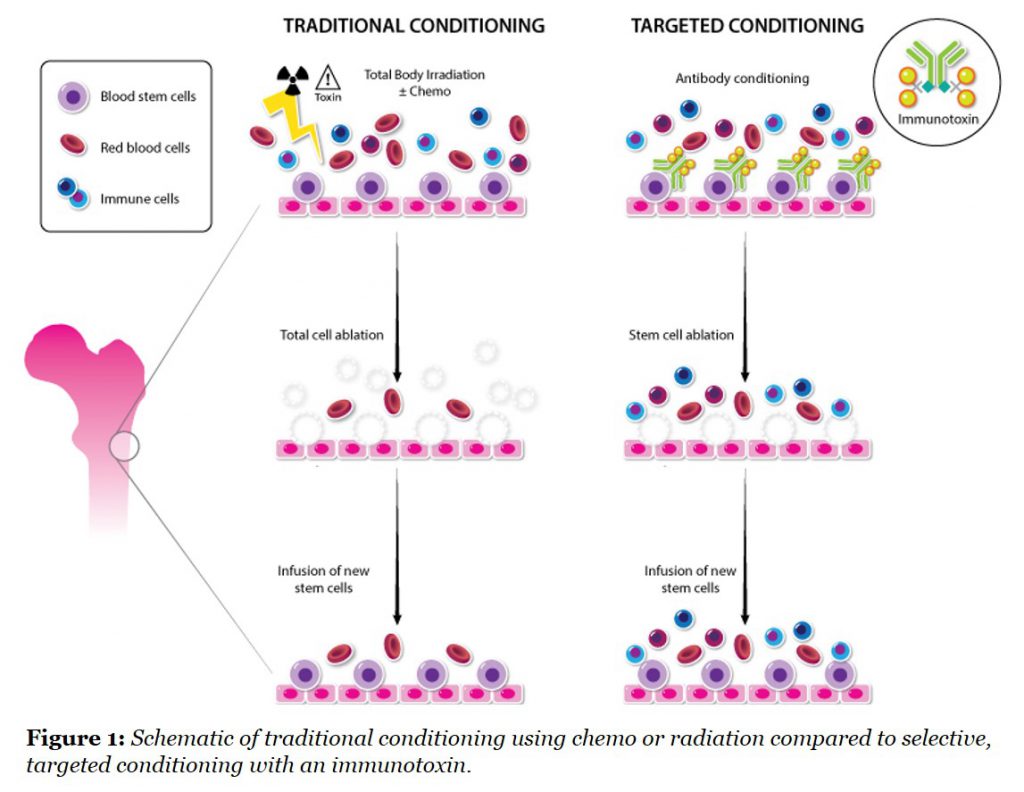
One major development that we are focused on was published recently by our Rahul and colleagues in Nature Biotechnology (here), and applies a technology from the tumor immunology field to stem cell conditioning for the first time. The group demonstrated that targeted antibody-toxin removal of stem cells from the bone marrow is a more effective and less toxic way to prepare animals for a transplant eliminating the need for radiation. The comparison of this new approach to the existing non-selective approaches is shown in Figure 1.
The use of more potent and selective agents for this purpose is relatively new in the stem cell field. When the CD45 antigen was targeted with an antibody coupled to a saporin toxin, the immunotoxin could effectively remove host stem cells, thereby making the “space” for a new stem cell transplant. Further studies showed that animals with sickle cell disease, which is caused by a mutation in bone marrow stem cells, could be cured by first conditioning with this CD45 immunotoxin followed by a transplant with normal stem cells.
There are numerous implications for this technology that were summarized in the companion review (here), written by another of our Founders, Luigi Naldini, M.D., Ph.D., particularly in genetic diseases and gene therapies. By removing the safety barrier, we could then consider patients with aggressive autoimmunity as candidates for transplant, a relatively underexplored but powerful strategy for rebooting the immune system. It was Alan Tyndall, M.D., another Magenta Founder, and colleagues who led the first transplant studies described earlier in this blog post. The potential to reboot a patient’s immune system with a transplant of their own stem cells could lead to a paradigm shift in clinical practice, if we eliminate the side effects that currently overshadow the tremendous benefit. We have a lot of work ahead to make this approach a treatment of choice for patients with immune diseases, but the latest clinical trials look very encouraging and show that the rampant course of aggressive multiple sclerosis and scleroderma can be completely halted in many patients treated in multi-center clinical trials.
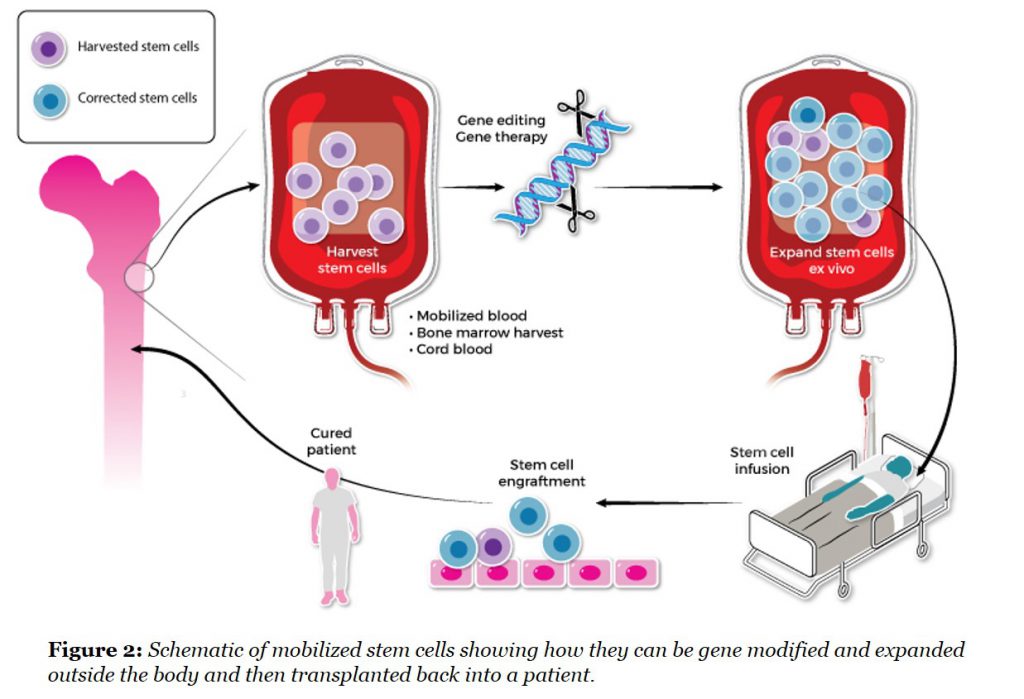
The other key component to a successful transplant is the medicine, which is fundamentally a question of stem cell quality and number. We have assembled parallel approaches to solving the challenges of stem cell harvesting which today either requires a surgical removal of donor bone marrow under general anesthesia or a one-week treatment with a growth factor, G-CSF, which moves (or mobilizes) stem cells from the marrow to the blood where they can be more easily collected. The discovery that G-CSF could mobilize stem cells in this manner was made serendipitously in the late 1980s (here) during its development as a drug for neutropenia, and this catalyzed the mobilization approach for stem cell harvesting. However, G-CSF may also cause significant side effects, particularly in autoimmune disease patients. At Magenta, we have identified new pathways that can be targeted to mobilize optimal stem cells in a more physiological manner and are excited by the early results in our preclinical models.
We also have a program that can expand the numbers of stem cells prior to transplant by targeting the self-renewal pathways that stem cells use. This has been a long-term goal for this field as clinical outcomes are directly correlated with stem cell numbers transplanted and many patients receive transplants with too few cells due to the collection challenges (here).
These approaches are particularly important for gene therapy and gene editing applications of stem cells where modified cell doses are crucial and often limited by the large amount of viral vector required for efficient transduction. A rapid way of expanding modified stem cells would quickly reduce the amount of expensive vector that is needed for each patient (See Figure 2).
The stem cell harvesting and expansion issues induce a vicious cycle that limits the number of available donors (who don’t want to go to the operating room for a bone marrow harvest) or can’t mobilize sufficient cells. This causes more unmatched transplants to be done in later stages of disease to compensate for these challenges. The non-matched donor stem cells can cause aggressive immune problems, notably graft-versus-host-disease, that can kill a patient and is further reason that the spectrum of disease settings for transplant has been limited.
Company background
For myself, I had worked on stem cells in the mid-1990s during a postdoctoral fellowship in David Scadden’s lab at Harvard. Like many scientists at that time, I had isolated them, differentiated them into different immune cells and even modified them with prototype gene therapy vectors. It would be just over decade later in industry, that I had the chance to build a stem cell discovery unit at GSK and would collaborate with David and Derrick Rossi as part of the Harvard Stem Cell Institute Alliance. In parallel, I had collaborated with John DiPersio on stem cell harvesting approaches and with Luigi Naldini to develop stem cell gene therapies for patients as part of an alliance with the San Raffaele Institute in Milan.
I was fortunate to work with leading physicians who had educated me on the promise and perils of stem cell medicine. Seeing the life-changing impact of a transplant on patients and their families is the type of opportunity that motivates many of us to go into a career in science. I knew that I wanted to tackle the challenges that have limited transplant and then apply this powerful treatment to more diseases.
But how?
Then I met Bruce Booth on a run around the Charles River with the #RunningAtlas crew on a brisk Thursday morning last Summer. “Atlas is interested in stem cell transplants – what do you think?” remarked Bruce shortly after the start. We ran, we talked and by the time I was showered and back in the office I had an email in my inbox asking if I would come on board as an Entrepreneur-in-Residence at Atlas to look at the area with the investment team. I knew David and many of the protagonists in the field, and was aware that Alexis Borisy over at Third Rock Ventures was also actively assessing the area. It seemed like a perfect opportunity and a few months later, I would join Atlas to create a company around this vision with Third Rock.
We had a lot of science to work through, but the first order of business was the business. Following multiple discussions, Atlas and Third Rock financed the Company officially as a joint seed project in November 2015. It was special opportunity for me to be working with two excellent early stage venture firms. Combining their two company-build approaches was powerful, and no doubt the subject of a sequel blog. Without an official name (we were called Project HSCTCo) but with a handful of part-time associates from each firm, a few consultants, and our academic founders rolling their sleeves up, we were off and running in the incubator at Atlas.
Over the year, we talked to over 100 external transplant experts and received tremendous feedback on what they wanted to change in clinical practice, discussed our immune reboot hypotheses with rheumatologists and neurologists treating patients with autoimmune diseases and started to test various plans. We built a business model, ran some validation studies to generate early data and challenged our assumptions with the investment groups at Atlas and Third Rock.
Along the way, we recruited the Magenta team. Many of the early members stepped up voluntarily, others came to us through our investors’ connections together with scientists from our Founders’ networks and labs. Over one month, Bastiano Sanna (COO) and Mike Cooke (CSO) joined up from Novartis, and with Christina Isacson making the seamless transition from Third Rock to Head BD, we had our full management team in place. With senior, experienced R&D leaders and scientists coming into Magenta during 2016, we were ready to migrate in the Summer to our own lab space in the Forsyth Institute in Cambridge to start generating our own data.
Throughout this formative period, I had weekly meetings with Bruce and Alexis to discuss science, tactics, business, investment milestones and it was a fast education for me in the venture creation world. We were joined by Mike Bonney, former CEO of Cubist, first as an advisor then transitioning to Executive Chair of the Board, and more recently recruited Tom Daniel as an independent Board Director shortly after his retirement as the President of Research and Early Development at Celgene. Our scientific and clinical teams coalesced around the new vision with David Scadden and Derrick Rossi, and we were joined by Alan Tyndall from the autoimmune transplant field, Luigi Naldini, a pioneer of stem cell gene therapy, together with leading hematology-oncology transplant physicians, John DiPersio (Washington University) and Robert Negrin (Stanford) as part of a stellar Founder group and Scientific Advisory Board. We remain very humble about the magnitude of the task ahead but are delighted with the teams that are driving Magenta. The R&D team is cranking out new data and the first SAB and BOD meetings were epic events. All key ingredients to a challenging and fun start-up experience.
That’s the story of Magenta. Like some babies, we took some time to decide on our name. Magenta is a spectacular color that does not exist in the normal visible light spectrum and is created by the chimeric combination of red and blue light. Chimerism is the hallmark of a successful stem cell transplant with the joining of the new (immune and blood system) in the old (patient), and this also felt emblematic of our combination of science and people that built Magenta.
We have enjoyed being in stealth mode for the past year, but with the American Society of Hematology conference next month and the opportunity to talk more widely about our vision and science within the autoimmune community, it felt like the right time to launch Magenta (Website). We are looking forward to the future as we execute on our vision to reboot the conversation between patient and doctor and at the same time use a stem cell transplant to clinically reboot patients’ immune systems to fight cancers, genetic diseases and autoimmunity.