2020 was an incredibly paradoxical year for those of us in biotech: the striking dichotomy of simultaneously experiencing agonizing tragedy alongside buoyant optimism.
It was truly a horrifically tragic year for humanity, and for America in particular. As everyone knows, the worst viral pandemic in a century has ravaged the country and taken over 350,000 American lives. Sadly, the desensitization of large parts of society to death and suffering is also alarming, including for those of us in biotech who are working to prolong life, and improve its quality, through better health. We’re witnessing a death rate comparable to the 9/11 disaster every day, and that event mobilized the entire nation for a decade. Today, while valiant efforts are being made, it’s hard not to feel the utter exhaustion of American society from the pandemic. It reminds me of the parable of the frog and the boiling water, it seems many have gotten desensitized to the dying happening around us – and that’s tragic.
Couple the pandemic and its horrendous toll on our lives and our consciousness to the other two major social traumas that afflicted us in 2020: a contentious and divisive election ripped through America, exposing deep cultural divides; and police brutality triggered protests across the country, mostly peaceful but some destructive, and re-ignited important discussions of systemic racism and inequity. Governments at the federal, state, and local level routinely mismanaged many of these crises in 2020. Much of the leadership has come from the private sector doing the right thing across many of these fronts.
Lastly, in 2020 the “real” economy on Main Street in America took a massive nosedive. Lockdowns and work from home orders shuttered many businesses, especially those in food, entertainment, and travel businesses. Millions lost their jobs, as unemployment spiked to record levels in the spring, though is steadily recovering. Our GDP dropped its largest quarterly decline ever in the spring as the coronavirus wreaked havoc across the country. Consensus estimates are that this real economy will take years to fully recover.
In light of this, many referred to 2020 as the ultimate dumpster fire of a year. Good riddance!
But herein lies the paradox: 2020 was also the greatest year ever for the biopharma industry, in particular for emerging biotech.
Science won the day in 2020. To quote Matt Damon in The Martian, “In the face of overwhelming odds, I’m left with only one option… I’m going to have to science the shit out of this.” Indeed that’s what the industry did. Science is leading us out of the pandemic. The COVID vaccines being developed and now deployed by multiple companies brought a constellation of biotechnologies together, evaluated them in enormous clinical trials, all in record time. Regulators helped facilitate their rapid development, while maintaining a high bar for efficacy and safety. Same goes for a number of new anti-viral antibodies and medicines. Despite the hype about whacky treatments, data from well-designed front line trials ultimately seems to have won – proving those things that worked, as well as proving that other candidate medicines didn’t work (and allowing us to cast aside ineffectual therapies). The positive sentiment for biotech out of the COVID response has been overwhelming, from society and investors alike. We obviously need to preserve that positive momentum.
But scientific progress wasn’t just about COVID.
Despite some of the R&D delays that occurred, particularly in the clinic, the R&D engine of the biopharma industry continued with amazing productivity and resilience. We are very fortunate to be in an industry that adapted quickly to the new environment, embracing the remote WFH and limited lab presence model. In many ways, it’s an extension of the virtual business model we’ve been establishing in drug R&D for the past decade or so. This more remote environment hasn’t diminished the productivity of the industry in a meaningful way, as evidenced by the pace of new drug approvals and exciting clinical progress.
The FDA’s CDER approved 53 new medicines in 2020, sharing 2nd place of all time with 1996 and a few approvals behind record-breaking 2018. This during a year when the FDA was massively strapped for the COVID response. Some great new medicines are now available for patients: Trodelvy for triple negative breast cancer, Danyelza for pediatric neuroblastoma, Risdiplam for SMA, and Tepezza for thyroid eye disease, to name a few. Many new medicines were approved for rare diseases again this year, like Duchenne, progeria, other others. Including rare cancers, more than two-thirds of recent approvals fit the rare disease definition. We even saw at least two infectious disease medicines for Ebola and Chagas.
Beyond these newly approved medicines, 2020 was full of other breakthrough clinical updates from the industry pipeline. Five of the most impressive later-stage outcomes include: CRISPR Therapeutics and Vertex’s CTX001 demonstrated exciting proof of concept data in sickle cell disease and transfusion-dependent beta thalassemia with CRISPR gene editing; Myokardia’s mavacamten delivered improved heart function in a Phase 3 study, leading to its acquisition by BMS; VelosBio’s VLS-101 showed impressive activity in heavily pretreated lymphomas, triggering Merck to engage; Novartis’ Iptacopan (LNP023) showed stellar Phase 2 activity in regulating complement in both C3 glomerulopathy and paroxysmal nocturnal hemoglobinuria; and, Acceleron’s sotatercept showed great promise in Pulmonary Arterial Hypertension.
Continuing their progress from 2019 (when I called them out initially in last year’s review), I’d also flag that Tyk2 continues to shine in psoriasis, SGLT2 continues to deliver in kidney and heart disease, and KRAS-directed programs in cancer continue to impress, all three of which revealed additional Phase 3 data in 2020.
These are just a few of the exciting R&D pipeline across the industry, but highlight the modality explosion that continues, as they represent gene editing and cell therapy, both active site and allosteric small molecules, antibody-drug conjugates, and next gen Fc-fusion biologics.
With this impressive scientific backdrop, it’s not a surprise that the biotech capital markets boomed in 2020. After collapsing like all of the equity markets in March 2020 as the reality of the pandemic hit hard, the biotech capital markets went on a tear for the next nine months.
In short, the biotech equity markets had their best year ever, bolstered in part by robust M&A activity. Here are the summary stats, with data courtesy of my good friends at Cowen & Company:
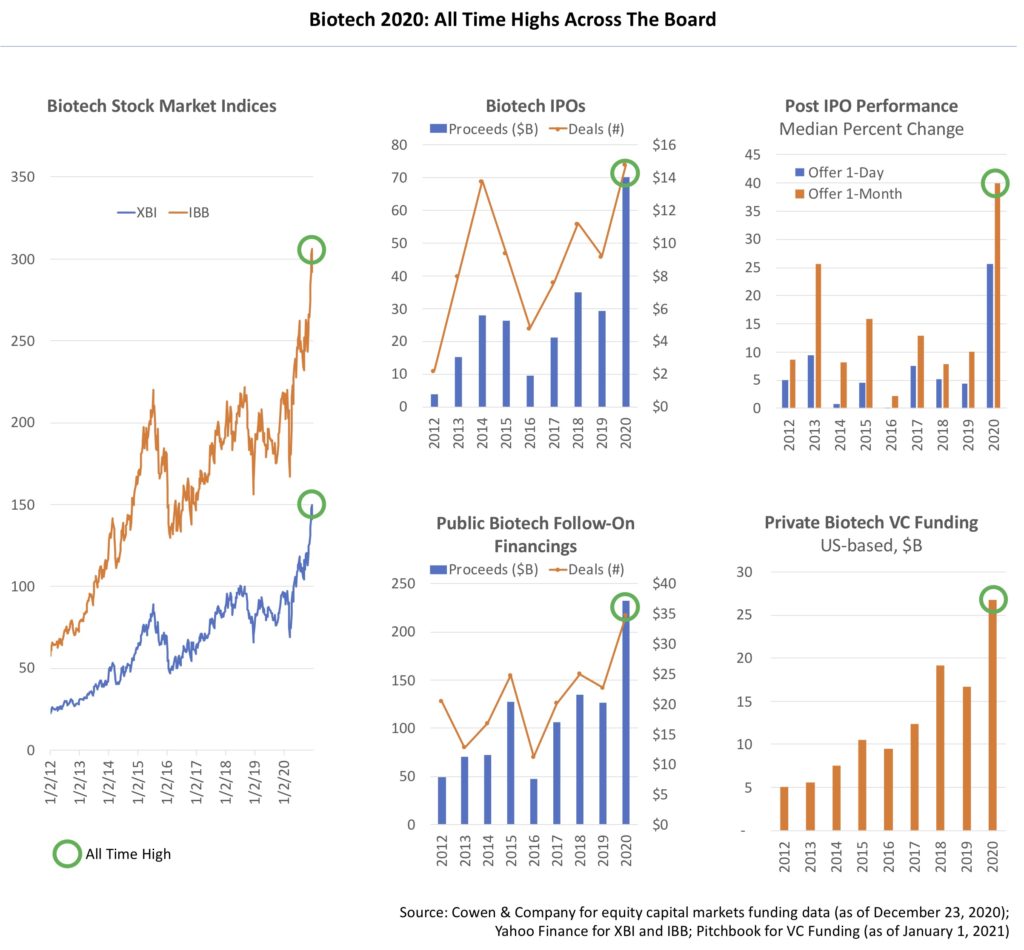
Every major biotech stock index hit its All Time High in 2020, and closed the year near the top. The equal-weighted SPDR Biotech ETF $XBI was up nearly 50% during the year, and up well over 110% since March, while the larger-cap biased NASDAQ Biotech Index was up ~25% and ~60% over those periods, respectively. These metrics outperformed nearly every other sector in the markets.
Biotech IPOs hit their All Time High for both volume and deal activity, raking in proceeds of over $14B across 74 deals. Median proceeds per deal doubled last year (up to $180M), with median market values approaching ~$500M. This level of IPO activity is nearly 2.5x more than in 2019, which was also a strong year for IPOs. The IPO market was simply on fire – a product of 2020’s unique context but also of a decade of evolution in the IPO marketplace itself. And this doesn’t even include all the SPAC IPOs that have been raised this year to acquire emerging biotech firms.
Post-IPO performance of the 2020 class with regard to first day and first month stock reactions also hit All Time Highs, with median performance up over 25-40% from the offer price – beating every year in at least two decades. Many of these new offerings have held onto those gains in the months and quarters after their IPOs. More than 80% remain above their IPO offer prices (here); for historical comparison, that’s usually much closer to 50-60% for an annual IPO cohort (related chart here).
Follow-on equity financings in biotech hit their All Time High as well, with 217 deals raising over $37B. This implies a staggering 4 deals were priced on average each week in 2020! The balance sheets of many emerging small cap biotech companies look incredibly strong right now, which bodes well for robust R&D funding in 2021-2022.
Venture capital funding in biotech not only hit an All Time High, but it blew the marker away. According to preliminary data from Pitchbook, over $26B of venture funding went into US-based biotech firms in 2020, with several quarters topping the charts for record-breaking funding (the “tsunami” in the first half continued into the second half of the year). 2018 was the prior high, and only hit $19B. This implies US-based private biotech firms were raising $500M per week in 2020! This is about 5x bigger than what we had at the start of the biotech bull cycle in 2013, a year that felt very robust at the time compared to the prior decade.
Further, while the equity markets were booming, the M&A market for both large and emerging biotech was also strong in 2020: AZ’s agreement to acquire Alexion for $39B, Gilead’s take out of Immunomedics for $21B, Myokardia’s acquisition by BMS for $13B, Momenta by J&J for $6.5B, just to name a few. Lots of smaller names in the early stage ecosystem were also acquired. As the equity markets soared, Pharma had to either step up and pay up, or miss out. This M&A activity is continued evidence of the resilience of the biopharma ecosystem, and created a “put” behind many small cap stock prices. This M&A support is clearly part of the equity market buoyancy.
Reflecting on these markets more personally, Atlas Venture also experienced some of the excitement in the equity capital markets over 2020 with a front row seat: over $3.7B in new capital came into our portfolio companies in 2020. We raised $600M across eleven seed and Series A financings, another $500M in Series B and later, four IPOs/SPACs raised $800M, and a dozen firms raised $1.8B in public follow-on financings. We also had four M&A exits, including Cadent and Vedere both by Novartis, Disarm by Lilly, and LTI by Bial. Thanks to the resilient entrepreneurs we work with, it was one of our strongest years ever, as summed up here in our Onward & Upward newsletter.
The 2020 Paradox and Thoughts On 2021
Stepping back from the celebration of these euphoric equity markets, it’s hard not to be jarred by the disconnect of biotech from the Main Street economy, with millions out of work, storefront businesses shuttering, and immense economic challenges across the board.
It’s a dumpster fire in 2020, but we’re hitting All Time Highs on every measure? Why is there this disconnect?
At least three reasons, several described in prior blogs (here, here).
First, biotech doesn’t follow conventional business metrics, and is therefore an odd part of the venture business ecosystem. Data is the ultimate currency of value in R&D-stage biotech, rather than traditional business metrics (e.g., MRR, CAC, etc…). While typical industries are often affected by acute changes in consumer demand (and spikes in unemployment), which alter their key performance metrics, this isn’t the case for biopharma in the short-term: we continue to push new medicines forward, as we did this year, generating the value-creating data that gives us confidence in progressing new medicines to market. Plus, with health insurance covering most medicines, the impact of acute economic changes on the actual sales of pharmaceuticals is relatively buffered. This creates a disconnect from conventional economic cycles, and is one of the reasons why biopharma tends to outperform other sectors during financial recessions (here).
Second, all of the equity markets bounced from March due to the flood of funds from the Federal Reserve. Massive monetary and fiscal support in the spring injected optimism into the equity markets, perhaps causing overbought situations in some sectors (like the WFH economy). With near zero interest rates today and for the foreseeable future, and investors seeking better returns, higher beta sectors like biotech often benefit in particular during these “risk on” periods. This has certainly been the case since the spring and continues today.
Lastly, the aforementioned preeminence of science, and the hugely positive sentiment towards biotech during this pandemic, has been a very strong tailwind driving demand for the sector. COVID-associated names clearly outperformed more generally, but the whole sector is contributing to the solid performance (here). Anecdotally, it’s also clear that retail interest has also flooded into the space, driving up demand for stocks across the board.
So where does all this put the biotech sector at the start of 2021?
All Time Highs are a tough place to start from a “high water mark” perspective, but there are plenty of reasons for optimism about the biotech markets as we head into the new year: science and biotech in particular continue to lead us out of the global COVID pandemic, strong R&D momentum around new medical innovations (including those with curative intent like gene and cell therapies), enormous dry powder on the balance sheets of emerging biotech companies, and an expectation of robust M&A as Big Pharma continues to leverage external R&D to add new biotech-discovered drugs to their pipelines.
But there are certainly a lot of risks. All Time Highs in the market are often followed by corrections or temporary pullbacks, as firms in the sector “grow into” their valuation expectations. We saw corrections in 2H 2015 and again in 4Q 2018, in the range of 45% and 30%, respectively. I don’t anticipate one in 2021, but there are a few possible triggers for a sector-wide pullback. A major unpredicted vaccine-related safety concern would be a big black swan negative, for both the sector and the COVID outlook. The gene therapy/editing space could see a deterioration in sentiment if further safety or durability issues arise with those approaches.
Another potential negative risk is that more dramatic drug pricing reforms are put forward by the new administration that hurt innovation (e.g., by pegging US medicines to depressed reference prices in Europe, for instance). An additional tail risk is if federal “march-in rights” around intellectual property are asserted, threatening the social contract around drug pricing, patent protection and their expiration. Tax policy changes that discourage long term investing would also be a negative for our sector. Many pundits seem to be discounting the impact of these policy areas, predicting the new administration will prioritize other areas in 2021, but politics has a way of surprising (to the negative).
Beyond the equity markets per se, I also worry about overfunding in the venture space: do we really have 5x better ideas than 5-7 years ago? Lots of lemming-like concepts are being funded: does the world really need another CD19-directed cell therapy with just a cute or subtle twist? I’m not sure. In other arenas of cancer research, the number of I/O-related programs and companies continues to expand quickly, though largely in the absence of differentiated efficacy? Other “hot” areas seem over-hyped relative to their practical application, with the most prominent being AI and Machine Learning in drug discovery. I’m sure better computational approaches will aid drug R&D, but we aren’t close to finding or designing new drugs purely in the computer and jumping straight to clinical studies.
While I’m an eternal optimist (which you have to be to some extent in early stage biotech), I’m also skeptical that the current prodigious amounts of capital being deployed today is exhibiting much productive discipline. The health of the herd, as I described here previously, depends on disciplined capital allocation, which requires an appreciation and understanding of the risks in our business and how to mitigate them. I’m just not sure the broader risks of “bleeding edge” science are fully priced into the capital markets today with regard to how those funds are being allocated.
Most importantly, will the billions in new capital translate into more drugs and thus more impactful value for patients and shareholders? This is the long term question that we’ll only know in hindsight.
I’ll leave specific 2021 predictions for the biotech market to others (see Brad Loncar’s thought-provoking list here), but feel confident in saying three things. First, with so much cash on the sidelines, there will be a lot of activity in the private and public capital markets (i.e. funding levels will be robust, albeit hard to hit All Time Highs again). Second, with a burgeoning industry pipeline of exciting new medicines, we’re bound to have a lot of both good and bad clinical news. And third, I expect lots of unpredicted macro and sector-specific curve balls, which will, as always, require us to change our forecasts – making these predictions moot.
After a paradoxical 2020, here’s to hoping for a great 2021!