This blog was written by Deanna Petersen, CBO of AVROBIO (@dmp9818), as part of the From The Trenches feature of LifeSciVC.
Building a leading gene therapy pipeline in under two years (and a lot of air miles): how to persevere and make the calls that matter.
Throughout a career in business development I’ve sat at every seat at the negotiating table, but I count my experience building AVROBIO’s leading lentiviral vector (LV) ex vivo gene therapy pipeline as perhaps my most satisfying and extraordinary time in biotech.
These last few years have underscored the awesome scope of the science that’s incubating in labs all around the world, waiting for the right team to develop and advance. My experience has also made me reflect on how much this business ultimately runs on relationships. Fortunes can (and do!) change dramatically with a single phone call.
AVROBIO: “COUNTRY WHEN COUNTRY WASN’T COOL”
My move to AVROBIO coincided with the beginnings of a sea change in medicine thanks to the life-changing promise of cell and gene therapies. I know this optimism is shared by many who have been impacted by rare diseases or cancer (including my daughter).
In 2015, I was serving at Shire as VP of business development in rare diseases, but I was starting to miss the challenge of building something from scratch with early-stage science that would fly under a big company’s radar.
In late 2015, I followed that feeling to become Chief Business Officer at AVROBIO. The lentiviral approach has heated up since then, but I like to think we were early fans — we were country when country wasn’t cool.
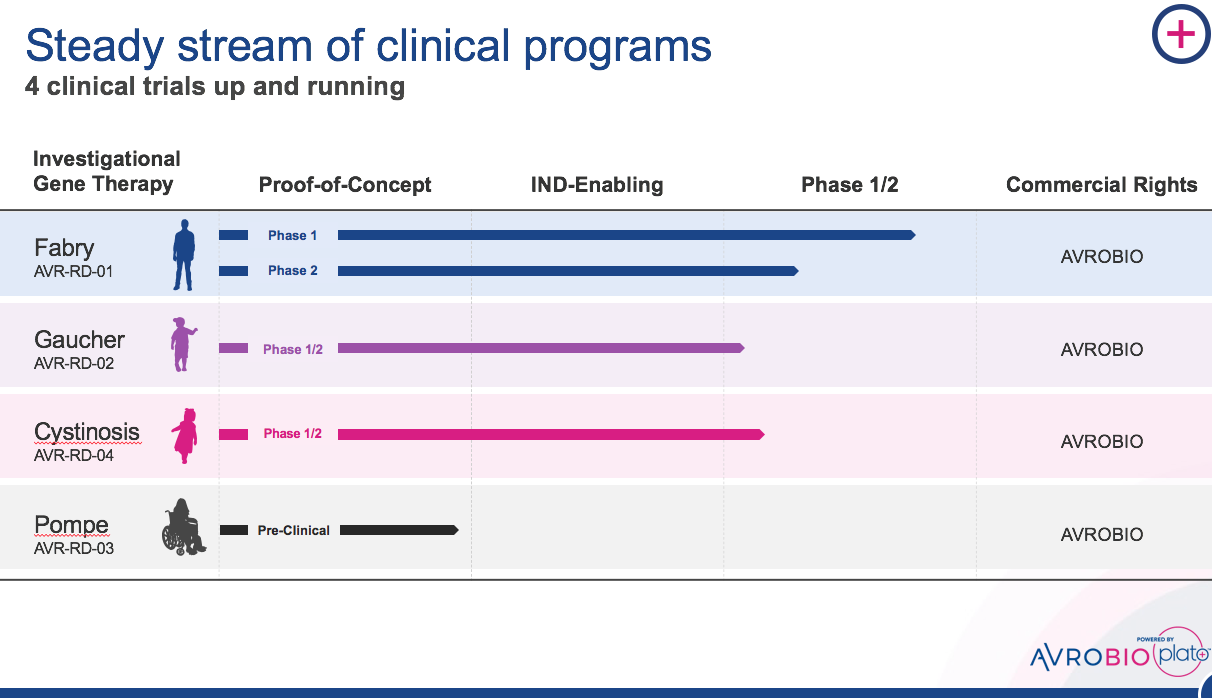
We have since evolved the LV approach, addressing historic development bottlenecks and delivering early clinical data in Fabry disease that provides promising support of our approach for a range of lysosomal storage disorders including Gaucher, Pompe and cystinosis. These are well understood monogenic disorders with known pathways to approval and significant room to improve on the current standard of care. We were hopeful we could make a big impact quickly, and our investors supported our clarity of focus.
We have already established an integrated development and manufacturing platform for LV – which we call “plato” – that includes three-day automated closed-loop manufacturing and a variety of efficacy optimizations (e.g. on vector copy number and transduction). Along the way we’ve completed an IPO, raised significant capital and attracted world-class talent to our team.
So how did we build a leading gene therapy pipeline in less than two years?
A GLOBAL SEARCH AND HIGH DRAMA
It all began with two preclinical lentiviral candidates on the table, one in oncology and one in Fabry disease. They had come to us from labs in Toronto as a non-negotiable joint bundle, prompting a crucial question: What kind of company do we want to be?
Cancer is an extraordinarily complex family of conditions, whereas Fabry is both very well- understood and has an established but sub-optimal standard of care, so an honest assessment about our expertise and mandate clearly pointed toward Fabry as the primary way forward. As it happened, I had previous experience with rare diseases, including Fabry, which further tipped the scales.
The identification of new candidates is hard work. Once the strategy was set, we were so fortunate to have a wellspring of ideas in the form of co-founder and Chief Science Officer Chris Mason. His deep immersion in the literature and wide network provided many leads for investigation, and at that point the stories often took a twist, as I recount below…
Gaucher disease was our second program. Chris found our candidate in a Swedish research program that was winding down. We had to move quickly, jumping on a plane and wiring them money to thaw mouse embryos and start breeding Gaucher mice again. We wanted to jump-start the experiments while we were still negotiating contracts! Then disaster struck – the postdoc who had done the proof of concept (PoC) work left the project for another role.
Suddenly, we were recruiters, headhunting the missing researcher and convincing her to come back to her own research project. After some negotiation, and some complex international contracting, she agreed to return and brought the project back to life. We breathed a sigh of relief.
But the relief was short-lived. As it turns out, professors (rather than their institution) in Sweden own their IP, which makes negotiating very complicated, so again our young program seemed in peril. We needed to get creative (and fast) with both American and Swedish lawyers to negotiate a technology license that worked for both parties.
We wore many hats in a short time. In fact, that whole program was a wonderful example of how the pursuit of new medicines requires the ability to rapidly become a temporary expert on many topics.
We continued to expand our lysosomal storage disease focus, looking next at Pompe disease. Treating this disease is a hard nut to crack as the enzyme is needed in difficult-to-reach target organs, including brain and muscle. Chris figured out a possible solution, namely, that using a GILT tag could help target our gene therapy to these cells and organs in a way that might deliver enhanced outcomes over competing AAV approaches. We were onto something special. But the GILT tag was proprietary to BioMarin. It’s a complicated process getting a major company to out-license their technology… but I happened to have the cell phone number of BioMarin’s head of BD. So I made the call, and a few months later, even though our initial internal champion at BioMarin had left, the deal got done.
After that adventure, we embarked on a global effort to find our next candidate, for cystinosis. We sent a scout to scientific conferences in Europe and the U.S. and our team was all over every imaginable channel looking for academic programs with a strong strategic fit. We certainly found them, though some were wrapped up and stored in research lab attics and basements.
We were really excited to find a cystinosis gene therapy program close to home, led by Dr. Stephanie Cherqui, an associate professor at University of California, San Diego and a champion for patients with cystinosis. Initially, Dr. Cherqui told us she wasn’t interested in partnering. We nonetheless sent a team out to meet with her in California; she politely took the meeting but reiterated that she wasn’t looking for a partner. A couple of months later, we tried again with a different team. Again, she politely took the meeting… and this time, we focused on discussing our common goal: getting this potentially life-saving treatment to patients as quickly as possible.
We brought a Gantt chart demonstrating the value of partnering from an early stage: we proposed that Dr. Cherqui could focus on running the Phase 1/2 clinical trial while we prepared for the pivotal study and ramped up commercial-scale manufacturing. Our shared commitment to getting this therapy to patients was the starting point needed to cement a a strong partnership with Dr. Cherqui, and we’ve had an excellent relationship ever since. The moral: sometimes it helps to keep knocking (politely).
We’re proud to announce that this week, the first patient in the Phase 1/2 cystinosis clinical trial has been dosed. It’s truly exciting and genuinely moving to be a part of the effort seeking to bring this investigational gene therapy to the cystinosis community.
After a rollercoaster ride, we’ve built a broad and diverse gene therapy pipeline, but it took some doing. I’ve reflected a lot on the experience and believe there have been a few essential ingredients to our success.
LESSONS LEARNED
Trust your expertise
The AVROBIO founding team included the former CEO of cell therapy leader Organogenesis, Geoff MacKay; renowned cell and gene therapy researcher (and gene therapy hunter extraordinaire), Chris Mason; and Kim Warren, a cell therapy manufacturing whiz. When I joined with a background in rare diseases, the choices we made were informed by a complementary set of experiences that gave us an edge in building our gene therapy pipeline.
Have a rock solid rationale for your focus
We knew from the start that we weren’t going to invent a whole new area or start from scratch in a new disease. It takes a very long time to build up the knowledge base around a disease (particularly true in rare diseases), so we laid out a careful plan to build our pipeline with well characterized and related diseases (where we already had experience), at least at the outset, so we had the potential to get to the clinic quickly and leverage the expertise we gained in one program to advance the next. We always suspected there would be a lot of operational synergies, and that’s exactly how it is turning out.
Be clear about your prospecting mandate
This flows on from understanding your focus: where will you look for your pipeline assets? At Shire, my mandate had been later-stage assets for acquisition: I never would have looked in universities for new candidates. At AVROBIO, our mandate opened up that whole area for prospecting. And it wasn’t as though these were only blue sky ideas: by in-licensing academic programs we had 10-15 years of academic work per program to capitalize on. We knew our job was to be shrewd technology hunters — scouts and miners of the early IP landscape. And that gave us and our backers clarity around priorities and what success would look like.
Be ready to seize opportunities
Everyone knows about negotiations and contracts, but luck and relationships have played a big part in helping us work through the many obstacles that have come our way. Flexibility, determination, persistence, an open mind and strong relationships are key to solving seemingly intractable problems, and every opportunity should be seized to advance your position.
Persevere
We are building AVROBIO at a great time for cell and gene therapy prospecting. It has been an incredible journey, and very fun, but certainly not for the faint of heart. I’ve always said that every deal has its story; that certainly rings true for me and for AVROBIO. And we’re not done yet. I am looking forward to the stories, and the discoveries, yet to come.