By Nello Mainolfi, PhD, Co-Founder, President and CEO of Kymera Therapeutics, as part of the From The Trenches feature of LifeSciVC.
The inherent ability to combine the power of genetic-like knockdown with the flexibility of small molecules; the power to drug classes of targets that have been elusive to other therapeutic modalities; the use of affinity rather than occupancy-based catalysis – the promise of these advantages has made targeted protein degradation one of the most exciting new therapeutic modalities of the past 30 years.
The first example of this concept, found in a later abandoned patent, illustrated the simplicity of the idea: a heterobifunctional (or monofunctional) small molecule binds to both a disease-causing protein and an E3 ligase and primes the ubiquitin-proteasome system for protein destruction. Craig Crews, Ray Deshaies and others (here and here) later developed this concept in the early 2000s, and helped advance this new modality with determination and elegance into the technology that we know today. Alongside the therapeutic considerations in the first paragraph, it is the conceptual simplicity of the mechanism, combined with the ability to specifically target a single protein, that makes this modality one of the most promising disease and target agnostic modalities in the biopharma industry.
While there are several drugs in this class currently in clinical development, our recent disclosure marks the first time that degrader proof-of-mechanism has been established in a randomized, placebo-controlled study. I would like to spend some time on why Kymera decided to own this challenge and the significance of this important milestone, both for Kymera and for the whole space going forward, and why I believe that the data disclosed by Kymera last week will change how we think about targeted protein degradation going forward (slides here).
When we founded Kymera, our vision was, and continues to be, focused on becoming a fully integrated biotech company that will discover, develop and commercialize a new generation of medicines based on targeted protein degradation. This is a unique opportunity; I often say to our new employees that this is a once in a lifetime opportunity, but it is also a responsibility. The responsibility is to own the challenges and the evolution of this technology, and to enable the most transformative therapeutic hypotheses to be translated into the clinic to impact patients and change the natural history of serious diseases. In fact, one of the most important fundamental beliefs at Kymera is that we are focused exclusively on targets where the degrader rationale can be clearly articulated and is rooted in biological differentiation versus other technologies.
Our choice to work not only in oncology, but also in immunology-inflammation and now in five other disease areas, was driven by several factors: high unmet medical need, large market opportunities, and compelling biology with unique opportunity to impact the disease with targeted protein degradation. But beyond those drivers, we believed that the complexity of the biology in these diverse disease areas would allow us to learn much more about the technology and would make us a better company for it.
The understanding and optimization of degradation profiles across multiple different immune cell types and tissues, for example, is something that we had to learn very early on in the life of the company and is a training ground that working solely in oncology does not provide. For example, at Kymera, we have spent a lot of time studying and solving for the impact on the degradation profiles of our drug candidates of target protein and E3 ligase expression levels, of the differentiated biology across diverse cell contexts and different distribution across tissues.
Our IRAK4 program checks all of our boxes of what we think makes the optimal target for targeted protein degradation: it targets a clinically validated signaling pathway impacting diseases with high unmet medical need, where key nodes have not been drugged or drugged well by any other modality, and where targeted protein degradation can provide a transformative therapeutic solution.
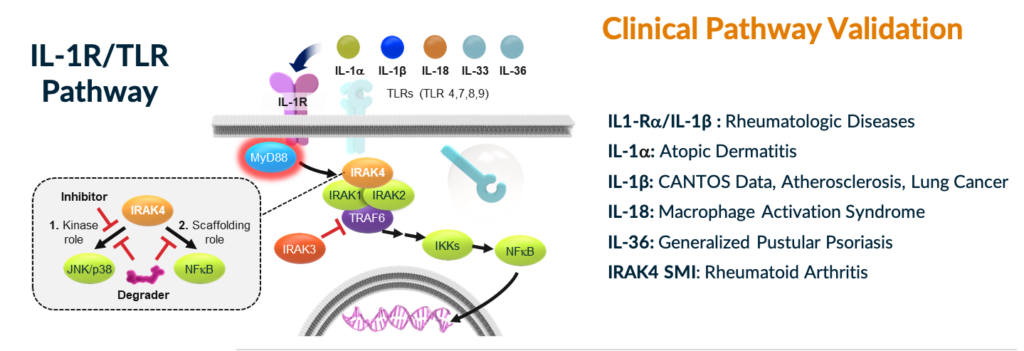
IL-1R/TLR Pathway and Targets with Clinical Validation
In fact, the IL-1R/TLR pathway has been clinically and commercially validated by several drugs in oncology, immunology, and inflammation. In particular, the role of the pathway has been demonstrated in several inflammatory and autoimmune diseases, including atopic dermatitis, rheumatoid arthritis, and other diseases. IL-1R/TLR signaling through the myddosome complex is dependent on IRAK4 kinase and scaffolding functions. Despite significant drug discovery efforts over the last two decades, there remains a need for a small molecule solution, with the convenience of an oral pill, to effectively drug this pathway intracellularly in order to provide an improved, broader therapeutic effect over single cytokine blockers. Small molecule IRAK4 kinase inhibitors, which have actually demonstrated positive clinical proof-of-concept, have been limited by only partially blocking signaling of this pathway through the kinase function, but not the scaffolding function, which regulates the stability and activity of the IL-1R/TLR-dependent MYD88 complex.
Degrading IRAK4 and fully blocking IL1R/TLR signaling is expected to be superior to antibody-based therapies and we also expect that a degrader will address the limitation of kinase inhibitors’ inability to blockade signaling through this pathway. Importantly, the safety of IRAK4 knockdown has been de-risked through human genetics, as IRAK4 null individuals are healthy adults. Hopefully, this helps in contextualizing the excitement we have around this target, and why I believe this moment is a key inflection point on the path to fully de-risking this asset towards being an important small molecule anti-inflammatory drug.
Based on our preclinical work, we expect approximately 85% degradation of IRAK4 will lead to clinical benefit that will be superior to other pathway blockers or small molecule kinase inhibitors. In preclinical species, we also saw that multiday dosing allowed us to achieve substantial levels of degradation (85% or more) once steady state degradation equilibria were reached.
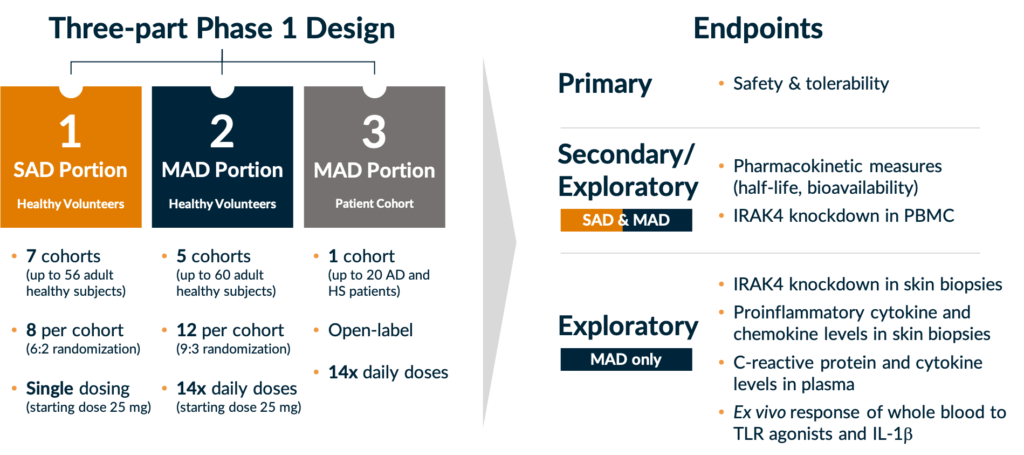
Our decision to run a Phase 1 study in a randomized, placebo-controlled format in healthy volunteers was driven by our development strategy in immunology and inflammation, but also by our desire to study the degradation profile of KT-474, our IRAK4 degrader, and elucidate the kinetics of single and multiple doses in order to determine the optimal regimen to take into patient studies. Importantly, we also wanted to generate a complete de-risking dataset by establishing KT-474’s pharmacokinetic and pharmacodynamic profile, as well as impact on disease relevant biomarkers in blood and skin, first in healthy volunteers and subsequently in patients.
KT-474 Phase 1 Trial Design
While targeted protein degradation has existed as a modality for over two decades, the study above represents several firsts for the field:
- KT-474 is the first heterobifunctional small molecule protein degrader outside of oncology to enter clinical development;
- It is also the first to be studied in healthy volunteers in a randomized, placebo-controlled study;
- And last week we reported the first proof-of-mechanism in such a study, which means the first time that a full and detailed PD profile including dose and time dependent degradation, has been reported in humans.
So, after 20 years, we feel privileged to break new grounds in the understanding of, and more importantly, in further developing this novel and potentially transformative therapeutic modality.
In our interim results announced on Monday June 28th, we observed for the first time the impact of dose and time on the pharmacodynamic effect of a potent, selective, and orally bioavailable degrader (in this case KT-474) in humans in a placebo-controlled manner:
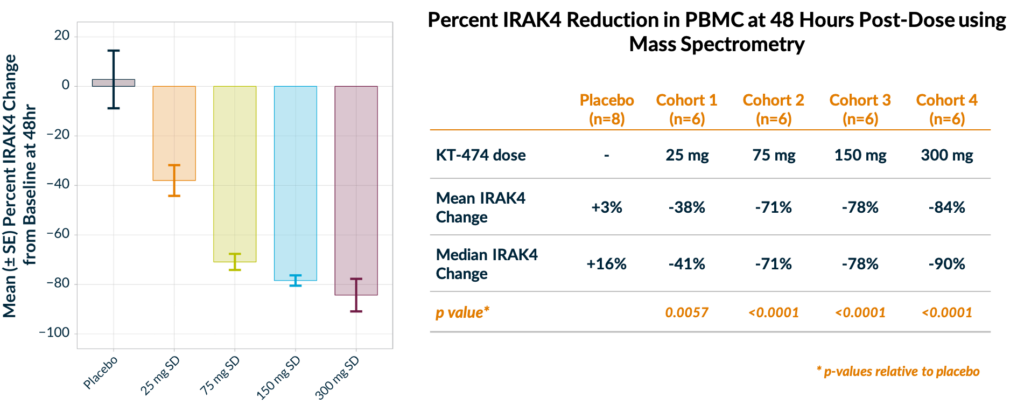
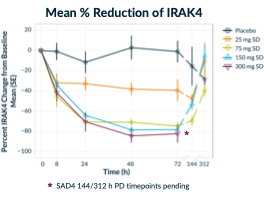 We have seen rapid target knockdown as early as 8 hours post-dose, with maximum reduction at 48-72 hours and degradation that lasts for at least 6 days after just a single dose without any treatment-related adverse events.
We have seen rapid target knockdown as early as 8 hours post-dose, with maximum reduction at 48-72 hours and degradation that lasts for at least 6 days after just a single dose without any treatment-related adverse events.- We have been able to reach and exceed our Phase 1 goal of 85% degradation, with 90% median and 94% maximal degradation at the 300 mg dose (IRAK4 levels were measured serially from isolated PBMC using mass spectrometry).
This collective dataset shows that we will likely be able to reach our therapeutically relevant target degradation levels upon repeat dosing and we will most likely be able to so at a lower dose. This is why we believe that this is such a key de-risking event for this program. These data also provide a key platform proof-of-concept, demonstrating that Kymera’s platform can generate highly potent, orally active, selective, and well tolerated drugs. So, with these encouraging data as a backdrop, we are very excited about our two oncology programs entering the clinic later in the year, along with our robust and emerging discovery pipeline across many different disease targets.
For the targeted protein degradation space in general, while this is interim single ascending dose data, the significance of these data speaks to the opportunity to explore other disease areas with orally active and selective drugs, the opportunity to optimize potency, pharmacokinetics, and distribution profiles to develop less frequent dosing paradigms for the right biological targets and hypotheses, and the opportunity to titrate degradation levels needed for particular target biology.
It is a fascinating coincidence that just two days before this dataset we saw first in class data also from another exciting new modality, with the first in vivo human mechanistic proof of concept for CRISPR editing. There are several parallels as well as obvious differences between CRISPR and Targeted Protein Degradation, but both modalities have the potential to transform treatment paradigms.
The combination of a genetic-like knockdown with the flexibility of small molecules (oral, systemic, widely distributed in human body) provides Targeted Protein Degradation with the potential to be target, organ and disease agnostic and truly expand the druggable genome beyond the approximately 20% or proteins drugged to date unlike any other modalities. The safety, PK, and time and dose responsive nature of the pharmacodynamic response shown above will hopefully accelerate the evolution of this transformative modality. We at Kymera are committed to doing so in order to develop transformative medicines for serious diseases that impact millions of patients around the world.