This blog was written by both Bill Marshall, CEO of MiRagen Therapeutics, and Ron Renaud, CEO of RaNA Therapeutics, as part of the From The Trenches feature of LifeSciVC.
Recent news from Alnylam, Ionis, and Mirna Therapeutics regarding toxicity-related setbacks in certain clinical programs have led many to ring the death knell for RNA-based therapeutic approaches. At the same time, an oligonucleotide-based approach to treating Duchenne’s Muscular Dystrophy (DMD) from Sarepta won an extremely controversial conditional approval from the FDA despite very limited evidence of efficacy.
This series of events has created an incredibly skeptical view of the field in general.
“…it has been widely believed that these molecules would be ideal reagents for imaging and therapy…however, the early excitement was rapidly replaced by disappointment when it became clear that these molecules were facing serious problems when used as therapeutics.”
One might well think that the quoted text came from a very recent article discussing the trials and tribulations encountered in nucleic acid drug development. In reality, it’s an excerpt from a 2009 review article discussing the challenges encountered in early development of therapeutic antibodies. And we all know how the therapeutic antibody story worked out.
The point being that any promising new therapeutic modality typically encounters early setbacks and requires iterative optimization of properties to become a mainstream tool in the pharmaceutical medical bag. This topic has been covered in an earlier “From the Trenches” article, but we thought with the recent setbacks being reported, it was appropriate to review the state of the field and the promise of the future.
A few bumps in RNA therapeutics road over the last few months have generated the usual hand-wringing about the future of siRNA, miRNA, antisense oligonucleotides and other related nucleic acid therapeutic approaches. Flashbacks have developed of 2011/12 when Novartis and Roche pulled out of RNAi, followed a few years later by Merck divesting its siRNA business. Some of the obituaries for RNA therapeutics that were written during that time have been recast. Admittedly, the RNA therapeutics space has always generated tremendous expectations with promises for rapid transition from in vitro to in vivo to the pharmacy shelf.
It seems so easy – identify sequences of mutated or disease causing genes, rationally develop an oligonucleotide with perfect complementarity (Watson-Crick base-pairing), administer that oligo to shut the aberrant gene off and the disease is ameliorated. This was the initial thesis. Unfortunately, the field has learned that there are a couple of Mt. Everest-like hurdles that prevents this fairly straightforward approach from being easily translatable. The hurdles for most nucleic acid based targeting strategies comes from the fact that, without significant chemical modification, the drug candidates do not have the most favorable pharmacological characteristics. Well-known Achilles’ heels for RNA therapeutic approaches include targeted delivery to specific cell types, short half-lives, instability, off-target toxicity and immune responses.
Fortunately, while all of the twists and turns have taken place over the last ten years, medicinal chemists have made great progress at knocking down many of these hurdles. Targeted delivery to the liver has been made possible with GalNAc conjugates. Other disease relevant cell types in the central nervous system, lymphatic system, cardiovascular system and in a variety of neoplasms can now be addressed by certain chemically modified oligonucleotides as well as nanoparticle formulations.
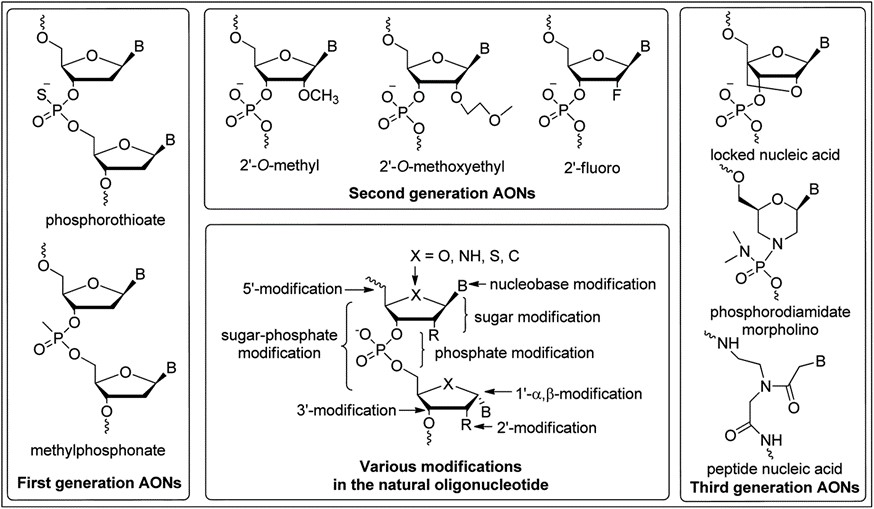
Major improvements to the pharmacological properties of nucleic acid drug candidates have come from how the nucleosides and internucleotide bonds are chemically modified. The phosphorothioate bond modification (sulfur replacement for an oxygen at the phosphate) has played a central role in the optimization of pharmacokinetics and bio-distribution. Many affinity enhancing modifications (Figure 1) to the backbone of oligonucleotides have focused on the 2’ position on the sugar. The most sophisticated of these modifications are the constrained nucleosides, which includes locked nucleic acid (LNA) and constrained ethyl (cET). Modification with these derivatives enables the use of smaller oligonucleotides with greater stability, affinity for their targets and cell penetrating activity.
Other well-established chemistries such as the 2nd generation 2′-O-methoxyethyl (2’MOE), have also led to greater affinity, potency and stability. The MOE modification was deployed on the first systemically administered antisense drug, mipomersen. A significant advancement in validation of the therapeutic approach.
One aspect of nucleic acid therapeutics that had been stressed for many years was the relatively consistent properties of a variety of candidate molecules that might be therapeutic development leads. The notion that one could screen a relatively limited set of molecules to find a potent modulator of gene expression, and that those molecules had consistent properties, added to the platform nature of the technology. This suggested an ability to rapidly produce drug leads. This was certainly a promising early hypothesis with some support for the relaitvely consistent behavior of the molecules. Unfortunately, the mantra of “the molecules behave the same” has the unfortunate effect of casting the same light when the behavior of the molecule causes a concern. Thus the entire modality is brought into question when any molecule encounters an issue.
However, as the field has conducted more and more drug discovery campaigns, there has been a recognition that what might have previously been considered “minor” changes to the molecular entity can lead to profound changes in drug like properties. These include the “fine-tuned” changes to the chemical structure of the core oligonucleotide structure, targeting and uptake enhancing conjugates, linkers attaching the conjugate, and constituents of lipid nanoparticles. So the take home message has become that we really need to think about each molecule as having unique properties. When one thinks about small molecule drug discovery, this is an inherent expectation.
Figure 1: Common oligonucleotide chemistries*
Despite all of the advances in oligonucleotide chemistry, there are still many unknowns on the in vivo pharmacology side of the equation. Unlike small molecules, which generally follow the Lipinski rule of five (RO5) to increase the chance of success (mw <500, lipophilicity or logP <5, hydrogen bond donors <5, hydrogen bond acceptors <10), oligonucleotides have no real set of rules. But the field is certainly working on building them (remember that Chris Lipinski devoted an esteemed 34-year career at Pfizer deriving RO5).
Experienced oligonucleotide drug developers need to pay close attention to oligo length, specificity of sequence and position of backbone modifications in order to try to avoid past observations of toxicity or decreased potency. Even with that precision, biology remains amazingly unpredictable. Specifically targeting any nucleic acid can generate unexpected on-target side effects based on exaggerated pharmacology that can only be unveiled in larger population studies. That layered on top of the potential for off-target structure-related effects, non-specific immune activation and unanticipated effects of delivery vehicles make the development of a successful RNA therapeutic look eerily similar to almost every other modality in drug development.
We believe that the field is on the cusp of great things. Biogen and Ionis have submitted a New Drug Application (NDA) for the treatment of Spinal Muscular Atrophy (SMA) with nusinersen. It looks like a nucleic acid therapy may provide life changing benefits for patients in need. And after all, that’s why we do this. Stay tuned for more.
* Source: Nucleic acid therapeutics: basic concepts and recent developments, V. K. Sharma, P. Rungta and A. K. Prasad, RSC Adv., 2014, 4, 16618